- here.">Questions are posted every Monday, Wednesday, and Friday, and keys are released one week after here.
- Reminder before you start: most questions are IChO level or beyond.
- Day - Mar 20, 2020
- Day - Mar 18, 2020
- Day - Mar 16, 2020
- Day - Mar 13, 2020
- Day - Mar 11, 2020
- Day - Mar 9, 2020
- Day - Mar 6, 2020
- Day - Mar 4, 2020
- Day - Mar 2, 2020
- Day - Feb 28, 2020
- Day - Feb 26, 2020
- Day - Feb 24, 2020
- Day - Feb 21, 2020
- Day - Feb 19, 2020
- Day - Feb 17, 2020
- Day - Feb 14, 2020
- Day - Feb 12, 2020
- Day - Feb 10, 2020
- Day - Feb 7, 2020
- Day - Feb 5, 2020
- Day - Feb 3, 2020
- Day - Jan 31, 2020
- Day - Jan 29, 2020
- Day - Jan 27, 2020
- Day - Jan 24, 2020
- Day - Jan 22, 2020
- Day - Jan 20, 2020
Questions are posted every Monday, Wednesday, and Friday, and keys are released one week after here.
Reminder before you start: most questions are IChO level or beyond.
Day - Mar 20, 2020
[IChO2020Prep-Q12] The beauty of the turquoise color of Lake Salda, where blue meets white sands, fascinates those who see it. Lake Salda, in the southern province of Burdur’s Yeşilova district, has been referred to as “Turkey’s Maldives” in recent years for its white sandy beaches and turquoise water. In fact, turquoise is an opaque, blue to green mineral that is a hydrated phosphate of copper and aluminum with the chemical formula of CuAl(PO)(OH)·4HO, and is known as a gemstone. The word turquoise dates back to the 17century and is derived from the French turquois, meaning “Turkish” because the mineral was first brought to Europe through Turkey, from mines in the historical Khorasan Province of Persia. Phosphorus, which is also in the structure of turquoise, is an essential part of life. Without the phosphates in biological molecules such as ATP, ADP, and DNA, we would not survive. Phosphorus compounds can be found in the minerals in our bones and teeth. With few exceptions, minerals containing phosphorus are in the maximally oxidized state as inorganic phosphate rocks, which are partially made of apatite, and they are today the chief commercial source of this element. Phosphate products are used as fertilizers in agriculture. They are also used in animal feeds, as a leavening agent in baking powder and flour, as an additive to beverages, and in pharmaceuticals. Industrial uses include water softening, rust proofing, fire proofing, in insecticides and detergents, and for the manufacture of elemental phosphorus.

Lake Salda
There are three important allotropes of phosphorus: X, Y, and Z. However, another form of phosphorus,W, also exists (given below). Xis a soft, waxy solid. It is exceptionally harmful and to a great degree reactive and also displays chemiluminescence. Crystals of X are composed of P molecules. Y is obtained by heating Xto 250 °C within the sight of daylight. It is nonpoisonous and odorless. Y does not show chemiluminescence. It exists as a polymeric solid. Z is produced from X under inert atmosphere. Z is the most stable allotrope of phosphorus and has a layered structure. W is a form of phosphorus that can be produced by day-long annealing of Y above 550 °C.
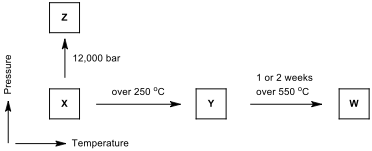
The interconvertible forms of all allotropes of phosphorus
12.1.Identify allotropes of phosphorus indicated by X,Y, Z, and W.
12.2. Draw the structure of X, Y, Z allotropes of phosphorus and sketch the geometry of X.
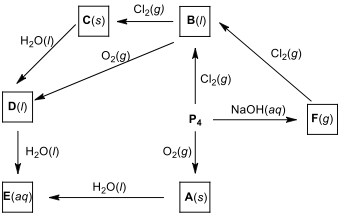
12.3. P ignites suddenly in air at around 35 °C to form a phosphorus oxide derivative. Thus, it is kept under water. When P reacts with different amounts of dry halogens, phosphorus trihalides (PX) or phosphorus pentahalides (PX) are obtained. PX can also be obtained by the reaction of the halogens with PX. The phosphorus pentahalides undergo hydrolysis in two steps to form acid. The phosphoryl halides can be prepared by the hydrolysis of the appropriate pentahalides in a limited amount of water or by the reaction of the trihalides with oxygen. Dropping of the oxide derivative of phosphorus into water produces a hissing sound, heat, and acid product. The reaction of P with sodium or potassium hydroxide produces phosphine gas as the major product and potassium or sodium hypophosphite as a by-product. Phosphine burns in chlorine spontaneously, forming a phosphorus trihalide (PX) or phosphorus pentahalide (PX).
Write the formulas of products A–F.
When phosphorus reacts with excess of halogens, it can form five-coordinated compounds such as PCl. Phosphorus mixed pentahalides like PFCl are prepared by the addition of one halogen to the phosphorus trihalide of a second halogen.
12.4. Draw the Lewis structures of PCl and PFClmolecules.
12.5. By using VSEPR theory, predict the molecular geometries of PCl and PFCl.
12.6. Estimate the polarity of PCl and PFClmolecules.
12.7. Compare the axial P–Cl bond length to the equatorial P–Cl bond length in PCl.
12.8. Draw the hybridization scheme of the PFClmolecule and estimate which hybrid orbitals are used to form the axial and equatorial bonds.
12.9.The synthesis of PHfrom hydrogen with white phosphorus is given below. Calculate ΔH for the following reaction, using bond energies (single bond energies (BE) (in kJ.mol) for P–P: 213, H–H: 435, P–H: 326).
Organophosphorus compounds are organic compounds containing phosphorus. Phosphorus can adopt a variety of oxidation states, and organophosphorus compounds are generally classified based on their derivatives of phosphorus(V) or phosphorus(III), which are the predominant classes of compounds. Organophosphorus compounds are widely used as nucleophiles and ligands. Two major applications are as reagents in the Wittig reaction and as supporting phosphine ligands in homogeneous catalysis. Their nucleophilicity is evidenced by their reactions with alkyl halides to give phosphonium salts. Phosphines are nucleophilic catalysts in organic synthesis, e.g., the Rauhut–Currier reaction and Baylis–Hillman reaction.
Triphenylphosphine (PPh) is a common organophosphorus compound and it is widely used in the synthesis of organic and organometallic compounds. When a toluene solution ofcompound 1 and excess of PPhare heated to reflux, first compound 2is formed and then compound 3**.
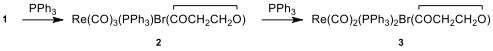
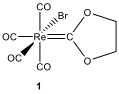
Spectral data of compounds 1–3 are given below (forH NMR and C NMR data [δ values (relative area)]: 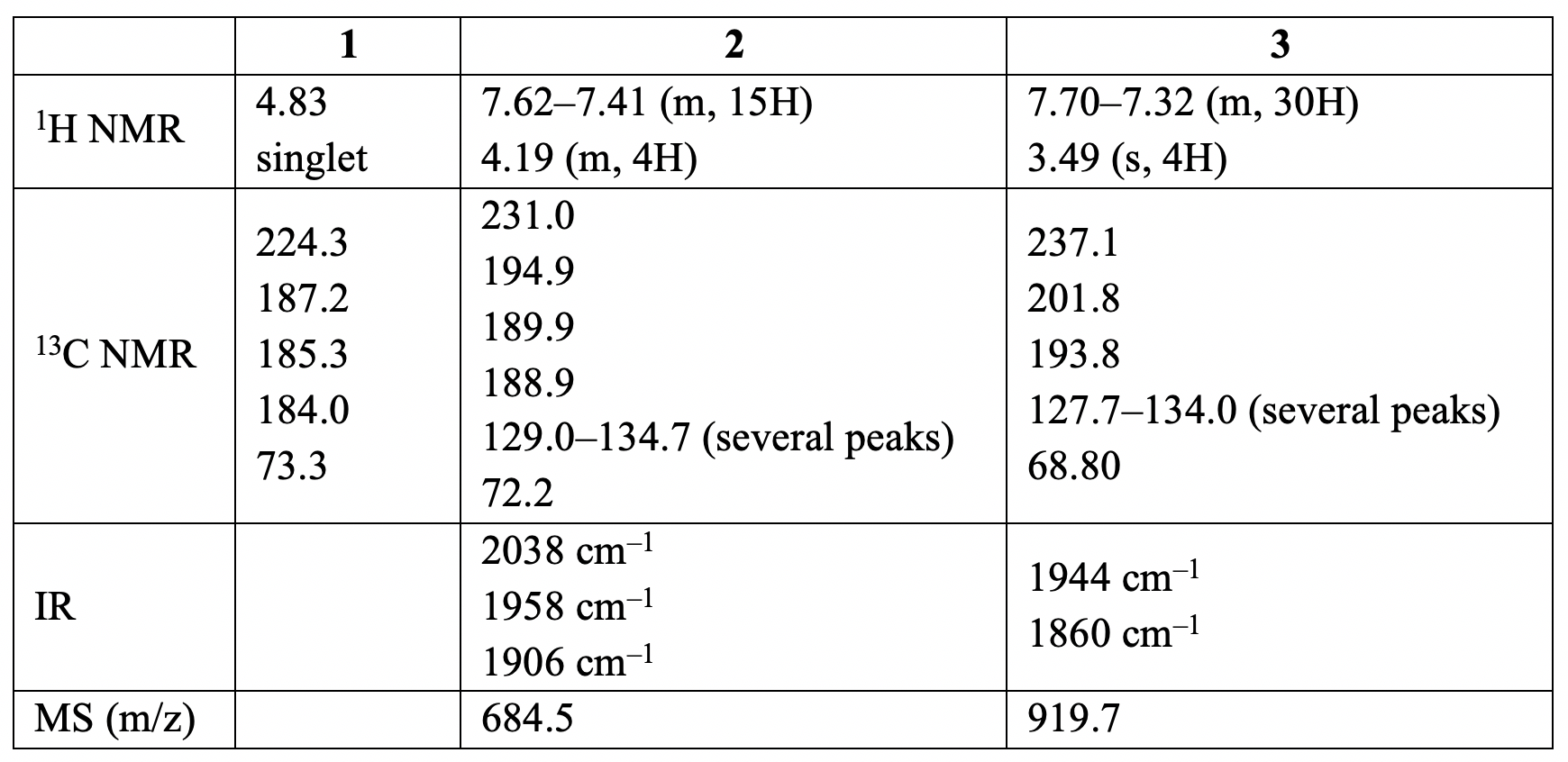
12.10. Identifythe structures of 2 and 3.
Hint: The C NMR signal of 1 at 224.3 ppm is similar to the chemical shift observed for carbene carbons; the peaks between 184 and 202 ppm correspond to carbonyls; and the peak at δ 73.3 is typical for CHCHbridges in dioxycarbene complexes.
12.11. Determine if 2is more likely to be the facial (fac) or meridional (mer) isomer.
Hint: The three ν(CO) bands with equal intensities are observed in the IR spectrum of compound 2. Protons of the carbene ligand occur as a multiplet in the H NMR spectrum.
12.12. Determine if 3 is more likely to be cis or trans isomer.
Hint: The two ν(CO) bands are of approximately equal intensity at 1944 and 1860 cmin the IR spectrum of compound 3. The P NMR spectrum shows a single resonance signal.
Some organophosphorus compounds such as sarin, soman, and VX are often referred as “nerve gases” despite the fact that they are liquids at room temperature. Each country signing the 1997 Chemical Weapons Convention agreed to ban the development of chemical weapons and to destroy chemical weapons and associated production facilities by 2012. Sarin can be destroyed by room temperature hydrolysis using aqueous NaCO to give NaF and the sodium salt of an organophosphate. The hydrolysis of nerve agent VX is more difficult. It reacts slowly with aqueous NaOH at room temperature, and the reaction has to be carried out at 360 K over several hours.
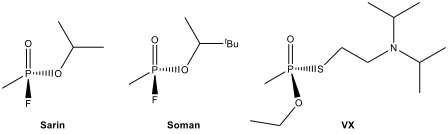
12.13.Determine the organophosphorus salt formed in the following hydrolysis reaction.
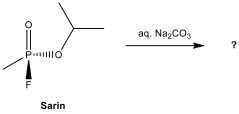
Two chromium complexes containing the ligands CO, PF, and PCl in octahedral geometry are given below. In an octahedral complex, the molecular orbitals created by coordination can be seen as resulting from the donation of two electrons by each of six σ-donor ligands to the d-orbitals on the metal, called σ-bonding. π-bonding (Pi bonding) in octahedral complexes is also possible when the ligand has p, d _or π_ molecular orbitals available. Ligands such as CO, CNand phosphines (of formula PR) are π acceptor, with empty orbitals that can interact with metal d orbitals in a π fashion. In most cases, the net back π bonding predominates, and electron density is transferred from the metal to the ligand. π-bonding can affect metal-ligand bond energy and bond length in carbonyl and phosphine complexes.
Answer the following questions considering the π interaction.
12.14. In which complex is the C–O bond shorter, Cr(CO)(PF) or Cr(CO)(PCl)?
12.15. In the infrared spectrum of which complex do the C–O stretching bands have *higher energy, Cr(CO)(PF) or Cr(CO)(PCl)?
Day - Mar 18, 2020
[IChO2020Prep-Q14] Medicinal inorganic chemistry based on metal‐based drugs is broadly defined as the area of research related to metal ions and metal complexes and their clinical applications. It is a new research area that developed from the discovery of the anticancer agent cisplatin. Cisplatin, cis-diamminedichloroplatinum(II), is a yellow powder and an anticancer drug widely used in the treatment of a variety of tumors, especially those of the testes, ovaries, head, and neck.
The synthesis of cisplatin starts with K[PtCl], but has undergone several improvements since it was published more than 100 years ago. The main problem is the occurrence of impurities and the formation of the by-product trans-platin. Nowadays, the synthetic routes are mostly based on a method published in the 1970s by Dhara. In the initial step, K[PtCl] is reacted with excess KI, and the platinum complex is converted into the iodo analogue (A). Subsequently, NH is added to the compound A and compound B is formed by ligand exchange in which two NH ligands are exchanged with two iodo ligands. Bis a yellow solid that is filtered, dried, and mixed with the aqueous solution of AgNO. The insoluble AgI can be filtered off and cis-diamminediaquaplatinum(II) nitrate (C) is formed; then excess KCl is added to the solution of C to yield cisplatin (D).
The success of the synthesis relies on the strong trans_effect of the iodo ligands. The spectator ligands T that are _trans _to the leaving group in square-planar complexes influence the rate of substitution. This phenomenon is called the _trans_effect. Key point is that a strong σ-donor ligand or π-acceptor ligand greatly accelerates substitution of a ligand situating in the trans position. _Trans _effects follow the order given below.
For a T σ -donor: OH- < NH < Cl- < Br- < CN-, CH-< I- < SCN- < PR, H-
For a T π-acceptor: Br- < I- < NCS- < NO- < CN- < CO, CH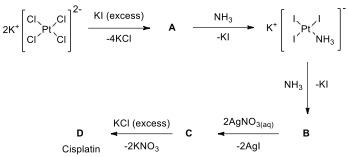
14.1. Write the formulas ofA–D.
14.2. Drawmolecular structures of A–D.
14.3. Is the complex Dpolar?
14.4. Sketch the d-orbital splitting of cisplatin complex D in view of Crystal Field Theory and show the electron distribution diagram.
14.5. Determinemagnetic nature of complex A.
The platinum complex binds to DNA and causes cross-linking, which triggers the programmed cell death (apoptosis). However, the other geometrical isomer of the square planar structure transplatin, _trans-diamminedichloroplatinum(II) (F), is not effective for the treatment of cancer. Transplatin is synthesized starting from [Pt(NH)] to which the first and second Cl ligands are added to form transplatin (F) as represented in the scheme below.
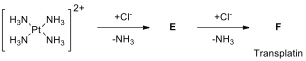
14.6. Draw the molecular structures of E and F.
The most important classes of antitumor agents, cisplatin, carboplatin, and oxaliplatin as platinum(II) diamines are widely used in chemotherapy to treat a wide variety of cancers.
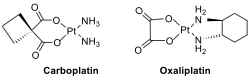
However, the therapeutic index of these agents is relatively narrow; their use is often plagued with severe toxicity and the development of resistance, which leads to disease progression. Recently, oxoplatin, iproplatin, ormaplatin and satraplatin are Pt complexes that have been used clinically (oxoplatin) or in clinical trials.
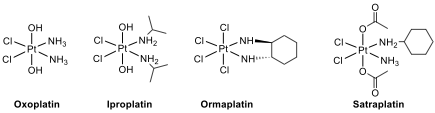
14.7. All complexes have the same geometry and oxidation number for the Pt central atom.
Writetheoxidation state of Pt and geometry of the complexes.
14.8. Which Pt complex, cisplatin or satraplatin, is kinetically more inert for substitution reactions?
14.9 Oxaplatin is an isomer of [Pt(NH)Cl(OH)] complex. Draw all stereoisomers and indicatethe chiral one(s).
Platinum complexes (oxoplatin, iproplatin, ormaplatin, and satraplatin) can be considered prodrugs that are primarily intracellularly activated by biological reducing agents such as thiols, ascorbic acid, and glutathione (GSH) to kill cancer cells.
In a study, for example, the reduction of cis,trans,cis-[PtCl(OCOCH)(NH)] (G, prodrug), which has a similar structure to satraplatin, by aqueous extract of cancer cells (A2780, A2780cisR, and HT-29) yields cisplatin (D, drug) and free acetate ion as given below.
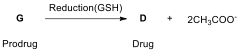
14.10. Drawthe molecular structure of G.
14.11. Sketch the d-orbital splitting of the metal ion in G and write the electronic configuration.
14.12. Decidewhether G is paramagnetic or diamagnetic.
14.13. The complex G crystallizes into a monoclinic crystal system of parameters: the lengths of the unit cell: a = 14.9973, b = 8.57220, c = 11.1352 Å, the β angle in the unit cell = 126.7690°, the number of the molecules in the unit cell (Z) = 4, M = 436.16 g/mol (the complex has one water molecule in the crystal structure).
Calculate the density (ρ) of the complex.
Hint: the volume of a monoclinic crystal unit cell is V=abc_sinβ_
Day - Mar 16, 2020
[Keeler2e-1.2] (a) Determine the oxidation number of nitrogen in NO.
(b) NO is known to be a free radical (i.e. it has an unpaired electron) and to adopt a bent geometry. One possible representation of the bonding in NO is shown below: indicate in the usual way the location of the electrons in this molecule, and verify that the formal charges shown are correct.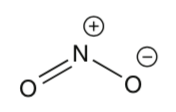
(c) Experimental data indicates that NO has a dipole moment which lies in the plane of the molecule and bisects the O–N–O angle. Explain why the structure shown above is not consistent with these data.
(d) Use the concept of resonance structures to give an explanation for the direction of the observed dipole in NO.
(e) Draw two alternative Lewis structures for carbon monoxide: one in which there is a triple bond and one in which there is a double bond. Determine if there are any formal charges on the atoms. Experimentally it is found that this molecule has a small dipole moment, with the carbon being δ. How can this observation be reconciled with your Lewis structures?
Day - Mar 13, 2020
[Keeler2e-9.7/8] Part 1. At high temperatures, calcium metal reacts with silicon to give a salt with empirical formula CaSi, and with carbon to give a salt with empirical formula CaC. X-ray crystallography reveals that the structures of these two salts are very different: CaC contains discrete C− ions,where as in CaSi the silicon atoms form sheets of(Si)n with each silicon having three Si–Si single bonds.
(a) Which element occurs in a form which is isoelectronic and isostructural with the Canion, and which element occurs in a form which is isoelectronic and isostructural with the (Si)_n _sheets?
(b) Why is there such a difference between the structures of the carbon and silicon anions?
(c) Calcium and silicon can also react to give a compound with empirical formula CaSi, whose structure is found to contain helical chains of Si atoms. How may the structure of these chains be rationalized?
Part 2. (a) In the anion PhC− the Ph-C bonds have a trigonal planar arrangement about the carbon, with the Ph–C–Ph bond angle being 120o. In contrast, in the anion Ph3Pb the Ph–Pb bonds have a trigonal pyramidal arrangement about the Pb, with a Ph–Pb–Ph bond angle of 91. Comment on the reasons why these two ions have such different structures.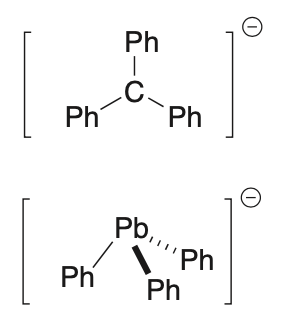
(b) From your answer to (a), explain why PhC− is a far stronger base than PhPb−.
Day - Mar 11, 2020
PhysC[ΙChO2017-Q3]
Part A.
Methanol is produced commercially by using a mixture of carbon monoxide and hydrogen over zinc oxide/copper oxide catalyst:
CO(g) + 2H(g) ⇌ CHOH(g).
The standard enthalpy of formation (ΔH) and the absolute entropy (S) for each of the three gases at room temperature (298 K) and at a standard pressure of 1 bar are given as follows.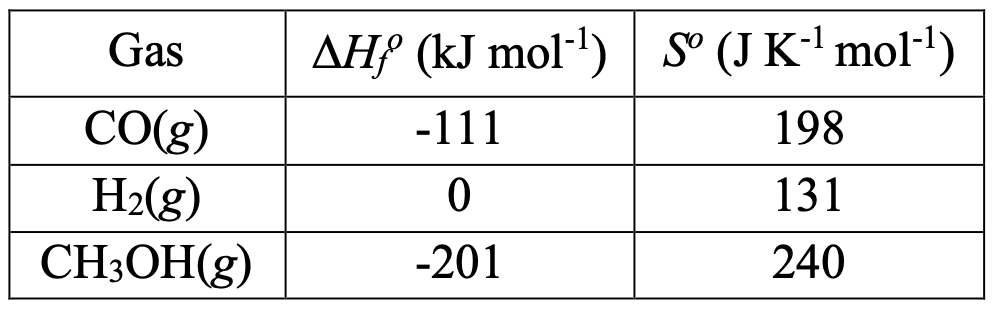
3-A1) Calculate ΔH, ΔS, ΔG, and K for the reaction at 298 K.
If you are unable to calculate K at 298 K in problem 3-A1), use K = 9 × 10 later on.
3-A2) A commercial reactor is operated at a temperature of 600 K. Calculate the value of K at this temperature, assuming that ΔH and ΔS are independent of temperature.
If you are unable to calculate K at 600 K in problem 3-A2), use K = 1.0×10 later on.
3-A3) Production of methanol in industry is based on flowing of the gas comprising 2.00 moles of H for each mole of CO into the reactor. The mole fraction of methanol in the exhaust gas from the reactor was found to be 0.18. Assuming that equilibrium is established, what is the total pressure in the reactor at a high temperature of 600 K?
Part B.
3-B) Consider the following closed system at 300 K. The system comprises 2 compartments, separated by a closed valve, which has negligible volume. At the same pressure P, compartment A and compartment B contain 0.100 mol argon gas and 0.200 mol nitrogen gas, respectively. The volumes of the two compartments, V and V, are selected so that the gases behave as ideal gases.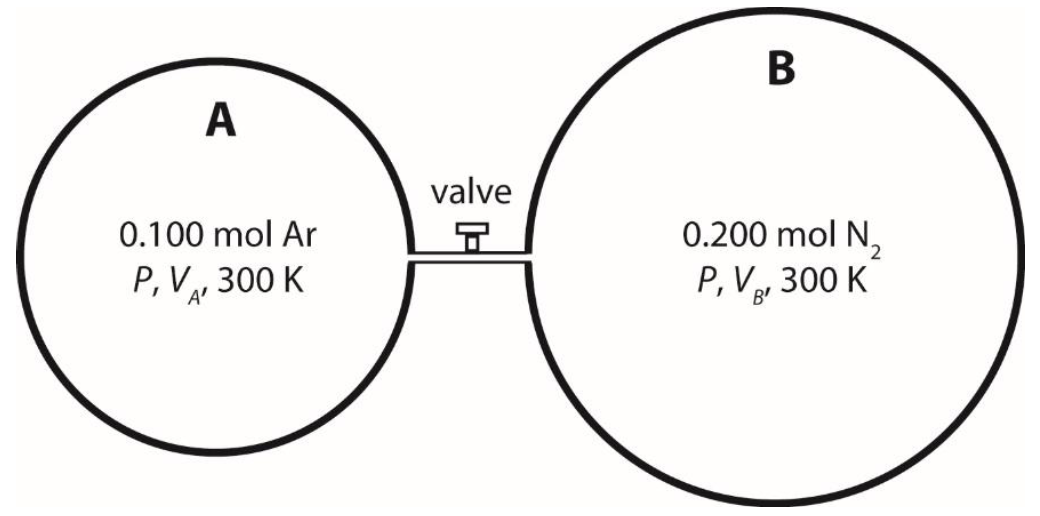
After opening the valve slowly, the system is allowed to reach equilibrium. It is assumed that the two gases form an ideal gas mixture. Calculate the change in Gibbs free energy at 300 K, ΔG.
Day - Mar 9, 2020
PhysC[USNCO2018-Q4] The air oxidation of an organoiridium compound (C9H11)3Ir takes place according to the following equation: (C9H11)3Ir + 0.5 O2 → (C9H11)3IrO
The reaction was studied with the initial [(C9H11)3Ir] = 1.3×10–4 M under two different O2 concentrations (run 1, [O2] = 1.0×10–2 M; run 2, [O2] = 2.0×10–3 M). The concentration of (C9H11)3Ir was measured as a function of time; below are plotted [(C9H11)3Ir], ln([(C9H11)3Ir]), and 1/[(C9H11)3Ir] vs. time.
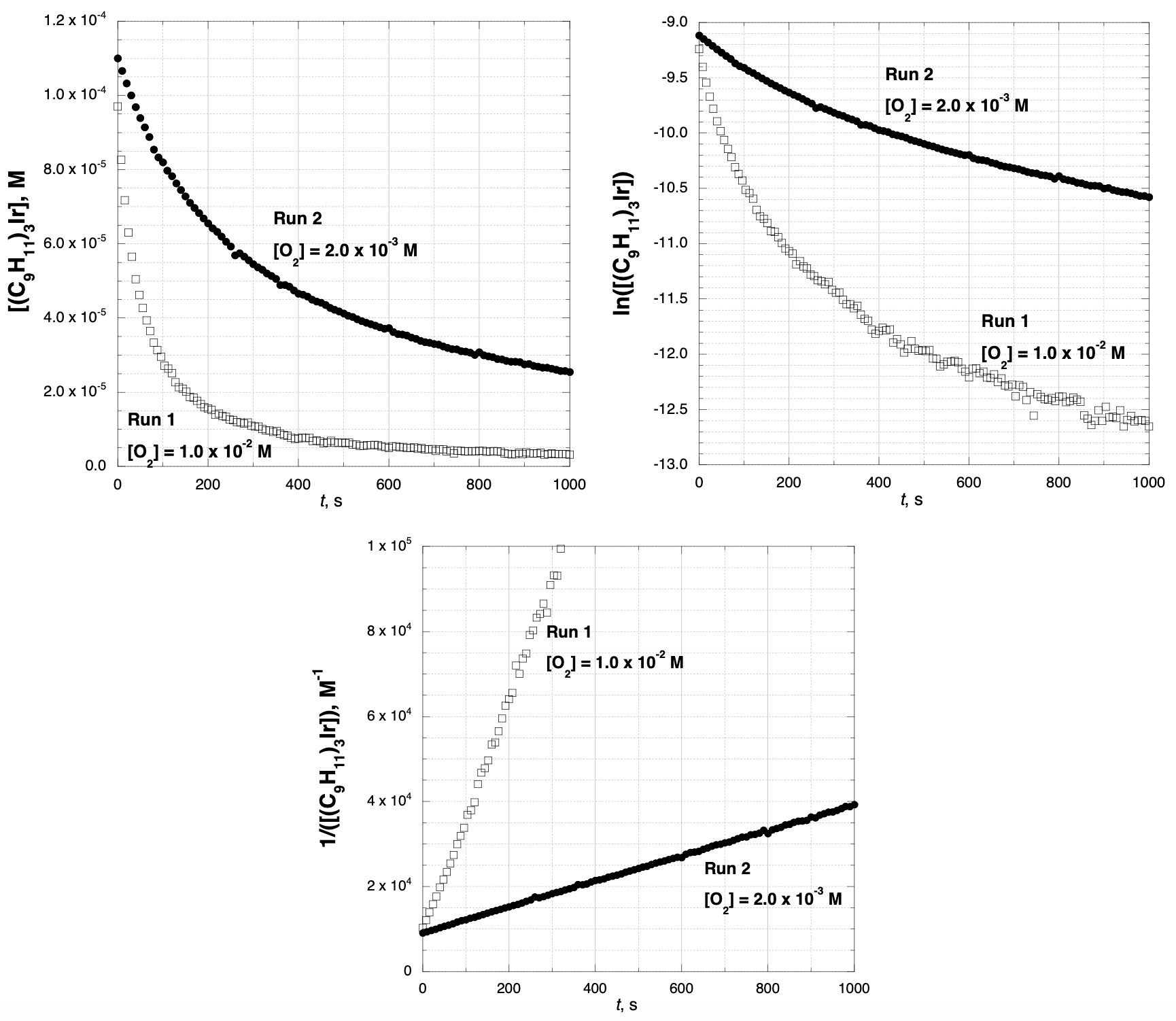
a. Is the order of the reaction in (C9H11)3Ir 0, 1, or 2? Justify your answer. (Even if the data are not exactly consistant with an integer order, pick the closest integer order.)
b. Is the order of the reaction in O2 0, 1, or 2? Justify your answer. (Even if the data are not exactly consistent wit order, pick the closest integer order.)
c. Calculate the rate constant for the reaction.
d. The following mechanism has been proposed. Is the mechanism consistent with the observed rate law? Explain reasoning.
Day - Mar 6, 2020
PhysC[NCChO2019-Q2] Under the catalysis of Mn(II), an organic dye (CR) solution is oxidized and faded by an oxidant (OX). The reaction has high sensitivity and selectivity under certain pH conditions. Based on this, a catalytic kinetic spectrophotometry can be established to determine trace manganese in water samples. The catalytic reaction is shown as follows:
The rate equation of the fading of CR is: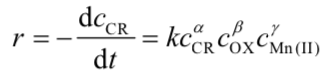
It is known that the fading reaction does not occur in the absence of Mn(II) in the system. During the reaction, the oxidant was greatly excessive.
(a) Fix the concentration of Mn(II) in the solution, change the initial concentration of the CR solution, and then measure the absorbance of the solution at a certain wavelength at 6.0 min and 9.0 min, respectively. Part of data are shown in Table 2-1, calcualte the value of α.
Table 2-1
| _c_CR (mol·L) | A(t = 6.0 min) | A(t = 9.0 min) |
|---|---|---|
| 2.0×10 | 0.274 | 0.111 |
| 2.2×10 | 0.333 | 0.135 |
| 2.4×10 | 0.482 | 0.195 |
| 2.6×10 | 0.669 | 0.271 |
(b) Experiments show that γ = 1. Derive a quantitative relationship between the ratio of the absorbance at two adjacent moments (A/Att) and the Mn(II) concentration at a fixed time interval Δt.
(c) Fix the CR initial concentration and the Mn(II) concentration, measure the absorbance at the same wavelength at t = 8.0 min and 10.0 min at 363.15 K and 373.15 K, respectively. The experimental results are shown in Table 2-2, calculate the observed activation energy of the reaction.
Table 2-2
| T(K) | A(t = 8.0 min) | A(t = 10.0 min) |
|---|---|---|
| 363.15 | 0.686 | 0.490 |
| 373.15 | 0.240 | 0.090 |
Day - Mar 4, 2020
AnalC[LCChO2001-Q13, with revision] The procedure for determining the purity of a phenol sample by potassium bromate is as follows: A 0.6000 g sample containing phenol is weighed and dissolved in 20.00 mL of a 0.1250 mol/L KBrO solution (the solution contains excess KBr), then further acidified with HSO(reaction 1). After the reaction is completed with no more white precipitate produced (reaction 2), KI(s) is added (reaction 3), and then the generated iodine is titrated with a 0.1050 mol/L NaSOsolution (reaction 4). 20.00 mL of titrant is used to reach the end point. Write down the net ionic equations of reaction 1, 3 & 4, and calculate the mass percent of phenol (CHOH, M_r = 94.11) in the sample.
Reaction 2: _2, 4, 6-tribromophenol is a white precipitate in aqueous solution.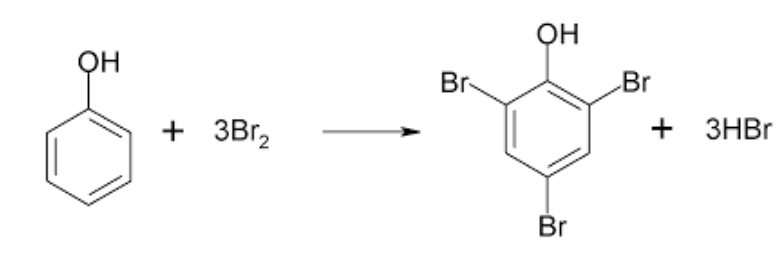
Day - Mar 2, 2020
AnalCPhysC[IChO2016-Q3] Iodine deficiency is of special concern in Georgia because it occupies a region where iodine is scarce in soil and water. Iodine deficiency can be effectively and inexpensively prevented if salt for human consumption is fortified with small amounts of iodine. Methods for analyzing salt for iodine content are thus important. Current regulations in Georgia are that iodized salt must contain between 25-55 ppm iodine (1 ppm = 1 mg iodine/kg salt).
Most salt is iodized by fortification with potassium iodate (KIO). Iodate content can be determined in salt samples using iodometric titration. In a typical procedure, 10.000 g of an iodized salt sample is dissolved in 100 cmof 1.0 mol/dm aqueous HCl to which 1.0 g KI has been added. The solution is then titrated with 0.00235 mol/dm aqueous sodium thiosulfate solution to a starch endpoint; this requires 7.50 cm of titrant.
(a) Write a balanced net ionic equation for the reaction when iodate reacts with excess iodide in acidic solution.
(b) Write a balanced net ionic equation for the reaction taking place during the titration with thiosulfate.
(c) Calculate the iodization level, in ppm, of this salt sample.
A less common agent for iodizing salt is potassium iodide, which cannot be easily measured by iodometric titration.
One possible method for analyzing iodide in the presence of chloride is potentiometric titration. However, this method is not very precise in the presence of large amounts of chloride.
In this method, a silver wire is immersed in the solution (containing iodide and chloride) to be analyzed and silver ion is gradually added to the solution. The potential of the silver wire is measured relative to a reference electrode consisting of a silver wire in a 1.000 mol/dm solution of AgNO. The measured potentials are negative and the absolute values of these potentials are reported. The solution to be analyzed has a volume of 1.000 dm(which you may assume does not change as silver ion is added), and T = 25.0 °C.
The results of this experiment are governed by three equilibria: the solubility of AgI(s) [K_I] and AgCl(s) [_K_Cl] and the formation of AgCl(aq) [_K]. (Iodide also forms complex ions with silver but this may be neglected at the very low concentrations of iodide present in this experiment).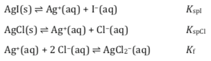
Below are shown the results of two experiments measuring the observed potential as a function of added number of moles of silver ion. Experiment A (solid circles) was carried out with 1.000 dm of solution containing 1.00∙10 mol/dmiodide and no chloride ion. Experiment B(open circles) was done using 1.000 dm of solution containing 1.00∙10mol/dm iodide and 1.00∙10 mol/dm chloride.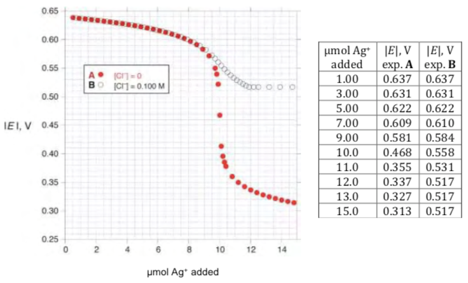
(d) Select an appropriate data point from the experiments and use it to calculate the solubility product of AgI (K_I).
(e) Select an appropriate data point from the experiments and use it to calculate the solubility product of AgCl (_K_Cl).
(f) Select an appropriate data point from the experiments and use it to calculate _K. You may need to use values of K_I or _K_Cl to do this calculation. If you were unable to carry out the calculations in (d) or (e), you may use the arbitrary values of _K_I = 1.00∙10 and _K_Cl = 1.00∙10 without penalty.
An analytical method that is more practical, because it is not sensitive to the presence of chloride, uses the Sandell-Kolthoff reaction. This is the reaction of HAsO with Ce(IV) to give Ce(III) in acidic solution, which is strongly catalyzed by iodide ion.
(g) Write balanced net ionic equations for the reaction of cerium(IV) with HAsOin acidic solution, as well as reactions of cerium(IV) with a species containing the element iodine and HAsO with a species containing the element iodine, that could reasonably account for the catalysis of the net reaction by iodide.
The reaction of Ce(IV) with HAsO can be monitored by measuring the absorbance at 405 nm, as Ce(IV) is orange and absorbs significantly at 405 nm, while the other reactants and products are colorless and do not absorb appreciably. Three runs were carried out, all in 0.50 mol/dmHSO at 25.0 °C using the following initial concentrations: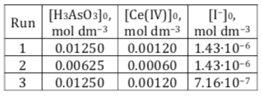
An analyst initiated the reactions by mixing the reagents in a cuvette. After a short variable delay absorbance measurements were started, with the first measurement recorded at _t = 0 s. The data obtained are shown below: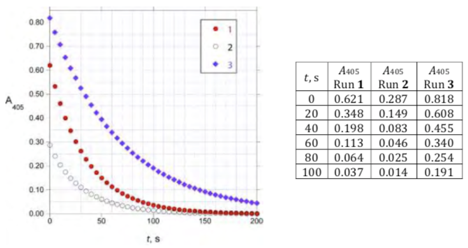
Under these conditions (0.5 mol/dm HSO, 25.0 °C), the rate law for the reaction can be written as
Rate = k[HAsO][Ce(IV)][I]_where _m, n, and p are integers.
(h) Determine the values of m, n, and p and calculate the value of k(be sure to specify its units).
A 1.000 g sample of iodized salt is dissolved in water to give 10.00 cmof solution. A 0.0500 cm aliquot of this solution is added to a mixture of 1.000 cm 0.025 mol/dm HAsOin 0.5 mol/dm HSO and 0.800 cm0.5 mol/dm HSO. To this mixture is added 0.200 cm 0.0120 mol/dm Ce(NH)(NO)in 0.5 mol/dm HSO and the absorbance at 405 nm is measured as a function of time at 25.0 °C: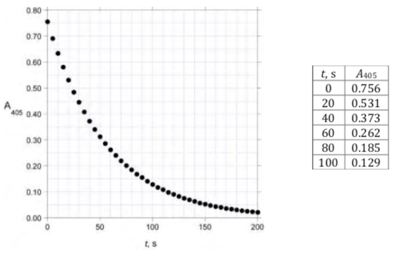
(i) Calculate the iodization level, in ppm, of this salt sample.
Day - Feb 28, 2020
PhysCAnalC[IChO2019-Q4] In the 19th century, the French entrepreneur B. Courtois specialized in the production of nitrate A(M**A(NO3)m), used for gunpowder. Initially imported from Asia, A was later produced from nitrate B(MB(NO3)n) using exchange reaction with compound C, obtained from algae.
1. Find the formulas of nitrates A and B knowing that they are anhydrous salts of alkaline or alkaline-earth metal (MA and MB). One of the nitrates contains no more than 1 w% of non-metallic impurities while the other contains 9 ± 3 w% of impurities. The content of metals MA and MB in the samples is 38.4 w% and 22.4 w% respectively. Support your answer with calculations.
To obtain A, 262.2 g of solid compound C were added to the solution containing 442.8 g of B. B is known to be in excess. As a result, 190.0 g of white precipitate D were formed and removed by filtration. The filtrate was evaporated, and the obtained solid mixture E was heated until the mass of the sample (containing only nitrites, NO2-) was constant. The only gaseous product was dioxygen: 60.48 L at 0 °C at 1 atm (dioxygen can be considered as an ideal gas).
2. Calculate the composition (in w%) of mixture E considering that it contained only compounds Aand B and no other impurities, and that C was taken in pure anhydrous state.
3. Determine the formulas of compounds C and D and write the balanced reaction equation between B and C.
In 1811, when working with algae ashes, Courtois observed that copper vessels were worn out faster than usual. While he was studying this phenomenon, his cat entered the laboratory and spilled the solution of concentrated sulfuric acid on the dry algae ashes: violet vapors instantly came out of the vessel (1, sulfuric acid is the oxidizing agent): iodine (I) had just been discovered! Iodine was the cause of the copper corrosion (2). However, because of the medicinal applications of iodine, Courtois opened a new manufacture to produce it by reaction of algae with chlorine (3).
Nowadays, iodine is prepared from the set of reactants (NO, I, H) (4) or (IO, I, H) (5). Write balanced equations for reactions 1–5**.
| Reaction 1 | |
|---|---|
| Reaction 2 | |
| Reaction 3 | |
| Reaction 4 | |
| Reaction 5 |
The solubility of iodine is very low in water but significantly increases when iodide ions are added.
Together they form ions such as triiodide, I3−:
I−(aq) + I2 (aq) = I3− (aq) (6)
Equilibrium (6) can be studied through the extraction of I with dichloromethane. Indeed, I− and I3− donot dissolve in organic solvents but I2 does and, when extracted, it is 15 times more concentrated in dichloromethane than in water.
The following experiment was performed. To prepare the initial solution, a few crystals of solid iodine were dissolved in 50.0 mL of an aqueous solution of potassium iodide (0.1112 g). Then, 50.0 mL of dichloromethane were added, and the mixture was vigorously shaken until equilibration. After phase separation, each phase was titrated by 16.20 mL (organic phase) and by 8.00 mL (aqueous phase) of the standard aqueous solution of sodium thiosulphate pentahydrate (14.9080 g in 1.000 L of solution) in the presence of starch. The process is schematically represented below:
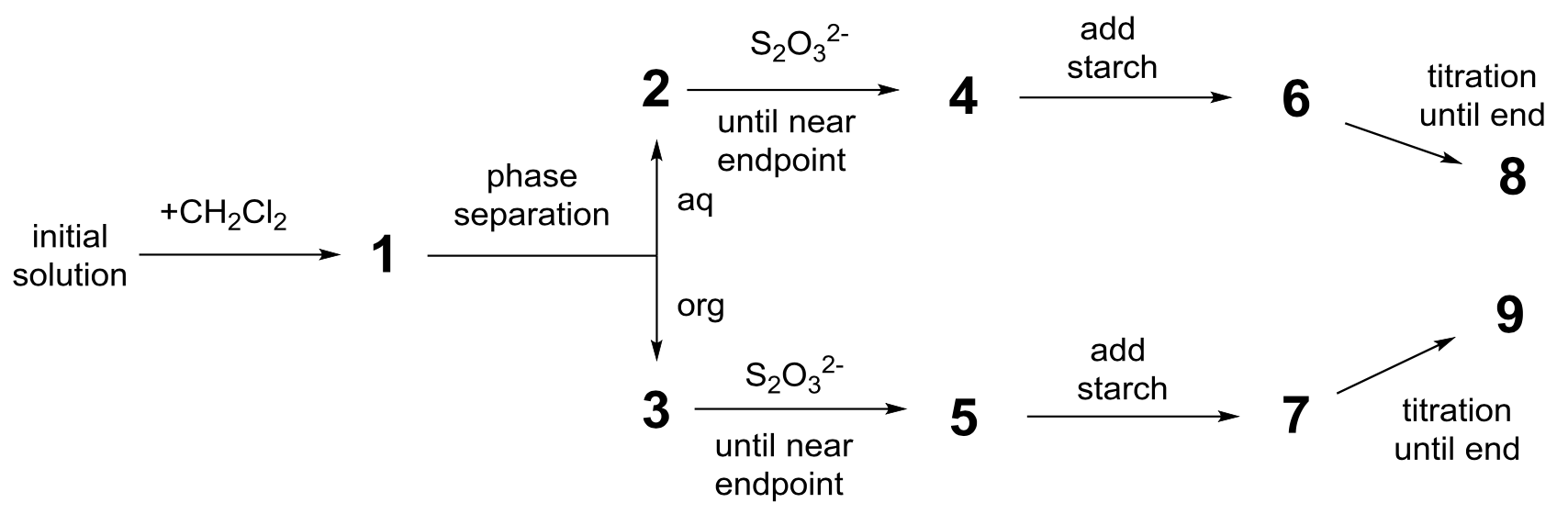
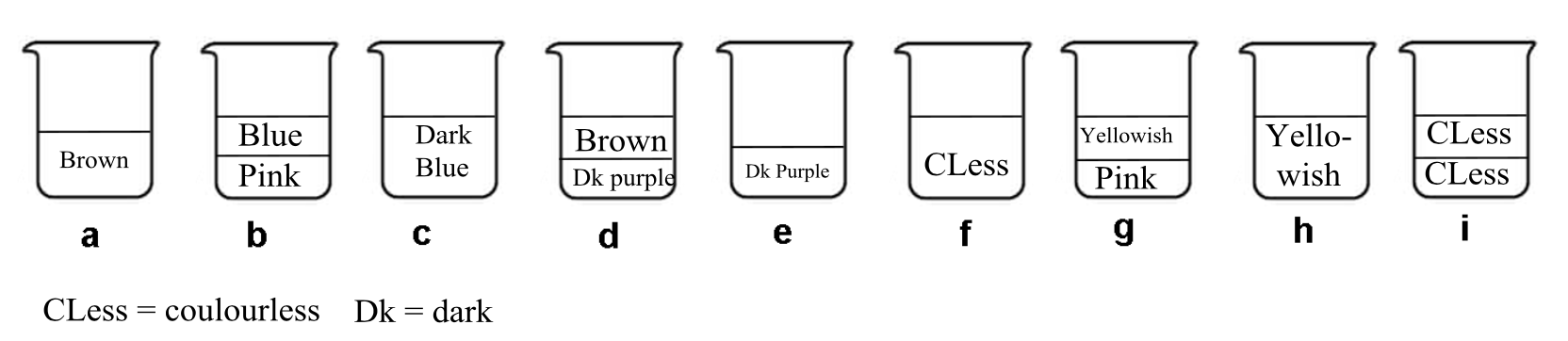
- Find the correspondence between the stages on the scheme (1–9) and the schematic pictures representing them (a–i).
| Stages | Picture |
|---|---|
| 1 | |
| 2 | |
| 3 | |
| 4 | |
| 5 | |
| 6 | |
| 7 | |
| 8 | |
| 9 |
- Write balanced equations for the two possible chemical reactions in the aqueous phase during the titration involving iodine species and sodium thiosulphate.
7. Calculate the mass of iodine used to prepare the initial solution.<br />8. Calculate the equilibrium constant _K_° for equilibrium of reaction (**6**).<br />
Day - Feb 26, 2020
PhysCAnalC[IChO2019-Q3] Data at 298 K: F= 96485 C/mol, R = 8.314 J·(mol·K)
pK(AgCl) = 9.7; pK(AgCrO) = 12
Formation constant of the complex [Ag(NH)]: β= 10
Potentials against the standard hydrogen electrode:
Standard potential of Ag/Ag(s): E°(Ag/Ag(s)) = 0.80 V
Apparent potential of O(aq)/HO(aq) (in seawater): E‘(O(aq)/HO(aq)) = 0.75 V
Part A: Quotes from a chemistry lesson by Louis Joseph Gay-Lussac
The following quotes from a chemistry lesson by Louis Joseph Gay-Lussac (French chemist and
physicist, 1778–1850) deal with some properties of silver chloride.
Quote A: “I will now talk about silver chloride, a milk-white solid. It is easily obtained by pouring hydrochloric acid into an aqueous solution of silver nitrate.”
Quote B: “This salt has no taste since it is insoluble.”
Quote C: “This compound is completely insoluble in alcohol and even in acids, except in concentrated hydrochloric acid which dissolves it readily.”
Quote D: “On the other hand, silver chloride is highly soluble in aqueous solution of ammonia.”
Quote E: “Then, we can make silver chloride appear again by adding an acid which reacts with
ammonia.”
Quote F: “If you take a bowl made of silver to evaporate salty seawater, you will get impure sodium chloride, mixed with a milk-white solid.”
(a). Quote A: Write the balanced chemical equation of AgCl(s) synthesis.
(b). Quote B: Calculate the solubility s of AgCl(s) in water at 298 K in mol L.
(c). Quote C: In a highly concentrated solution of chloride ions, a well-defined complex of
stoichiometry 1:2 is formed. On the following qualitative axis (with pCl increasing from left to
right), place in each domain the silver-containing species that is predominant (or exists, for
solids). pCl values at frontiers are not expected.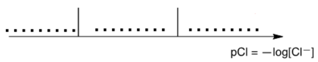
Quote D: When ammonia is added to silver chloride, a well-defined complex of stoichiometry n is formed.
(d). Write the balanced equation corresponding to the synthesis of the complex [Ag(NH)]from silver chloride and calculate the corresponding equilibrium constant.
If you could not calculate K, the following value can be used in the rest of the problem: K = 10
(e). Ammonia is added to 0.1 mol of silver chloride in 1 L of water until the last grain of solid disappears. At this moment, [NH] = 1.78 mol L. Determine the stoichiometry of the complex neglecting dilution effects.
(f). Write the balanced chemical equation corresponding to quote E.
(g). Assuming that seawater is slightly basic and rich in dioxygen, and that silver metal can reduce dioxygen in such conditions, write a balanced chemical equation corresponding to the formation of the solid mentioned in quote F. A stoichiometric coefficient of 1 will be chosen for dioxygen. Calculate its equilibrium constant at 298 K.
Part B: The Mohr method
The Mohr method is based on the colorimetric titration of Cl− by Ag+ in the presence of potassium
chromate (2K+ , CrO42- ). Three drops (~ 0.5 mL) of a K2CrO4 solution at about 7.76∙10-3 mol L-1 are added to V_0 = 20.00 mL of a sodium chloride solution of unknown concentration _C_Cl. This solution isthen titrated by silver nitrate (Ag+, NO3-) at _C_Ag = 0.050 mol L-1 , which immediately leads to the formation of solid A. A red precipitate (solid B) appears at _V_Ag = 4.30 mL.
(h). Write the balanced equations of the two reactions occurring during the experiment. Calculate the corresponding equilibrium constants.
(i). Identify the solids A and B.
(j). Calculate the unknown concentration _C_Cl of chloride ions in the sodium chloride solution.
_If you could not calculate CCl, the value CCl = 0.010 mol L−1 can be used in the rest of the problem.
(k). Calculate the minimal volume _V_Ag(min) for which AgCl(s) precipitates.
(l). Calculate the residual concentration [Cl−] precipitate. Justify why CrO42− is a good titration endpoint indicator by comparing two values.
Day - Feb 24, 2020
AnalC[MIChO2013R2-Q2]
Benzoic acid (PhCOOH) can be extracted from aqueous solution using benzene. The partition coefficient of PhCOOH in two phases is K (organic phase / water sample). The ionization constant of PhCOOH in the aqueous phase is K, and the equilibrium constant for forming a dimer in the organic phase is K.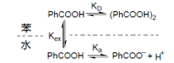
1 Draw the structure of the benzoic acid dimer.
2 According to the above diagram, provide the expressions of K, K, and K, respectively. The subscripts “a” and “o” are used to indicate the aqueous and organic phases, respectively.
3 Which of the following substances will increase the degree of extraction when added to the benzoic acid aqueous solution. Select from HSO, HCl, NaOH and HO, and briefly explain the reason.
4 In order to determine the above-mentioned equilibrium constant, the following three groups of experiments were performed: benzoic acid was extracted from a solution of V = 100 mL and c = 0.100 mol/L using different volumes of benzene, and the pH value of the aqueous solution after extraction was measured. As shown in the following table:
| Experiment | Volume of benzene used V/mL | pH of the aqueous solution after extraction |
|---|---|---|
| 1 | 0.00 | 2.61 |
| 2 | 50.0 | 2.73 |
| 3 | 100 | 2.80 |
Calculate the equilibrium constants K, K, and K.
5. Calculate the extraction yield R of benzoic acid with the same volume of benzene (Experiment 3). The extraction yield R represents the percentage of the benzoic acid extracted to the organic phase (including various forms) to the total amount of benzoic acid.
If you cannot calculate the constants in 4., please use K = 1.02×10, K = 1.74, and K = 2.23 to answer this question, the same applies below.
6. After passing HCl(g) to 100.0 mL of 0.100 mol/L benzoic acid aqueous solution until [H] = 0.100 mol/L and then extracting benzoic acid with an equal volume of benzene, how will the extraction yield R change? Calculate the extraction yield R in this new case.
7. What is the minimum amount of benzene needed to extract R = 90% of benzoic acid from the solution described in 6.?
Day - Feb 21, 2020
AnalC[LCChO2007 - Q6] Under certain experimental conditions, the partition coefficient (K) of a monoprotic weak acid HA in benzene (B) and water (W) is 1.00:
K_D=[HA]/[HA]=1.00
The analytical concentration of HA in water and benzene is 3.05×10 mol/L and 3.96×10 mol/L, respectively. In water phase, HA dissociates in the following way:
HA ⇌ H + A _K=1.00×10
And in benzene phase, HA conducts dimerization:
2HA ⇌ (HA)
1 Calculate the equilibrium concentration of all of chemical species in water phase and the pH.
2 Calculate the equilibrium constant (K) of dimerization of HA in benzene.
3 There is a benzene ring in the structure of HA, and there are 3.85×10molecules in 1.00 g HA. Derive the name of HA.
4 Explain why HA conducts dimerization in benzene by drawing the structure of the dimer.
Day - Feb 19, 2020
Inorgo[IChO1980-Q3] (Chemistry of ions, stoichiometry, redox reactions)
A white crystalline solid compound A exhibits the following reactions:
1) The flame of a Bunsen burner is intensively yellow coloured.
2) An aqueous solution of A is neutral. Dropwise addition of sulphurous acid (an SOsolution) leads to a deep brown solution that is discoloured in the presence of excess of sulphurous acid.
3) If an AgNO solution is added to the discoloured solution obtained by 2) and acidified with HNO, a yellow precipitate is obtained that is insoluble on addition of NH, but can be readily dissolved by adding CN or SO.
4) If an aqueous solution of A is treated with KI and dilute HSO a deep brown solution is formed that can be discoloured by addition of sulphurous acid or a NaSO solution.
5) An amount of 0.1000 g of A is dissolved in water, then 0.5 g KI and a few cm of dilute HSO are added. The deep brown solution formed is titrated with 0.1000 M NaSOsolution until the solution is completely discoloured. The consumption is 37.40 cm.
Problems:
1. What elements are contained in the compound A?
2. What compounds can be considered as present on the basis of reactions 1) to 4)? Calculate their molar masses.
3. Formulate the reactions corresponding to 2) to 4) for the compounds considered and write the corresponding equations in the ionic form.
4. Decide on the basis of 5) which compound is present.
Day - Feb 17, 2020
AnalC[LCChO2006-Q5] A solution of KI(0.100 mol/L)-I is titrated by 0.100 mol/L NaSO solution, and the measured c(I) = 4.85×10 mol/L. Mix 50.0 mL such KI-I solution and 50.0 mL CCl(l) in a separation funnel and shake the mixture until reaching partition equilibrium. The aqueous and organic phases are separated, and the c(I) in CClphase is 2.60×10 mol/L.
The partition coefficient (K) of I in CCl (O) and water (W) is 85:
_K_D=[I]/[I]= 85
Calculate the equilibrium constant of I+ I ⇌ I.
Day - Feb 14, 2020
OrgoPhysC[MIChO2013R1-Q2]
PhysC[IChO2018Prep-Q5] The main problem with studying ultrafast reactions is mixing the reactants. A smart way to circumvent this problem is the so-called relaxation technique.
Neutralization is a good example of an ultrafast reaction:
Here, k and k are the rate constants for the forward and backward reaction, respectively. The
mean enthalpy for this reaction is −49.65 kJ mol in the temperature range 298–373 K. The
density of water is 1.000 g cm.
1. Water has pH = 7.00 at 298 K. Calculate the apparent equilibrium constant 𝐾 =[HO]/([H][OH])
of the neutralization reaction shown above. Calculate also the entropy change for the reaction.
2. Estimate the pH of boiling water (T = 373 K).
Heavy water undergoes an analogous neutralization reaction, yet it is less dissociated than light
water at the given temperature: K(DO) = 1.35 × 10 at 298 K.
3. What is pD of heavy water at 298 K?
4. Write the rate law for the change of the concentration of DO in terms of the concentrations of D, OD and DO.
The composition of the equilibrium system depends on temperature. If we apply an external stimulus, for example a very fast heat pulse on the system, we disturb the equilibrium and observe a subsequent relaxation to the equilibrium composition. We can describe the relaxation with a new quantity x, a deviation from the equilibrium concentrations:
𝑥 = [DO] − [DO] = [OD] − [OD] = [D] − [D]
5. Express the time change d𝑥/d𝑡 in terms of x. Give both the exact equation and the equation
in which you neglect the small terms of x.
Solving the equation derived in 5., we get: 𝑥 = 𝑥(0) × exp(−𝑡×(𝑘[D] + 𝑘[OD] + 𝑘))
where 𝑥(0) is the deviation from equilibrium at the moment of perturbation.
6. For heavy water at 298 K, the relaxation time 𝜏 (time at which the deviation from equilibrium
drops to 1/𝑒 of the initial value) was measured to be 162 µs. Calculate the rate constant for
the forward and backward reaction. The density of heavy water is ρ = 1.107 g cm and
molar mass is M = 20.03.
Ultrafast reactions can also be triggered by a pH jump. Using an ultrafast laser pulse, we can
induce a pH jump in a system with so-called photoacids. These compounds have dramatically
different acid-base properties in the ground and excited electronic states. For example, the pK
of 6-hydroxynaphthalene-2-sulfonate is 9.12 in the ground state and 1.66 in the excited state.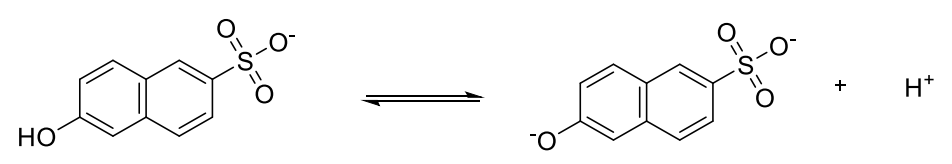
7. 1 cm of 5.0 × 10 mol dm 6-hydroxynaphthalene-2-sulfonate solution was irradiated by
light with the wavelength of 297 nm. The total absorbed energy was 2.228 × 10 J.
Calculate the pH before and after irradiation. Neglect the autoprotolysis of water in both
cases.
Note that the standard state for a solution is defined as c = 1 mol dm and assume that
the activity coefficient is γ = 1 for all species. It may be of advantage to use an online cubic
equation solver.
Day - Feb 12, 2020
Orgo[IChO1996-Q7]
Stereochemistry of organic compounds can sometimes be determined by studying their chemical behavior. The stereochemical configuration of one of the isomers of 5-nonbornene-2,3-dicarboxilyc acids (compound X)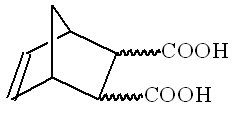 (no stereochemistry is shown)
(no stereochemistry is shown)
was established by the following experiments.
On heating this substance decomposes producing water and a new compound Y. Compound Y slowly dissolves in excess of aqueous NaOH with the formation of product X same to that is formed in the reaction of X with NaOH. The resulting solution of X is treated by I to give compounds containing iodine. Acidification of the solution leads to a mixture of two isomeric compounds, A and B in the 3:1 ratio. The titration of 0.3913 g of compound A by 0.1000 M aqueous solution of NaOH in the presence of phenolphthalein takes 12.70 mL of alkali. The same amount of 0.1000 M solution of NaOH is required for the titration of 0.3913 g of compound B. On heating compound A slowly transforms into compound C, which contains no iodine and is able to react with water under a certain condition (revision). Under the same conditions compound B does no undergo this transformation, but on heating with hydrochloric acid slowly transforms into A**.
All reactions must be written as balance equations. No mechanisms is required.
1. Mark by asterisks () the asymmetric carbon atoms in the structure of 5-nonbornene-2,3-dicarboxilyc acids.
2. Draw the stereochemical formulas of all stereoisomers of compound X, and the structures of products of their dehydration in those cases when it is possible.
3. Write the reactions of NaOH with a stereoisomer of X and a stereoisomer of Y.
4. Calculate the molecular mass of compound A. Write the reactions leading from X to A.
5. Write the reaction of the formation of C from A and the reaction of C with water.
6. Draw the stereochemical formula of compound X, which satisfies all of the data given in the problem.
7. Write the reactions leading from B to A.
8. Are the compounds A and *B diastereomers?
Day - Feb 10, 2020
AnalC[LCChO2007-Q6] Under certain experimental conditions, the partition coefficient (K) of a monoprotic weak acid HA in benzene (B) and water (W) is 1.00:
K_D=[HA]/[HA]=1.00
The analytical concentration of HA in water and benzene is 3.05×10 mol/L and 3.96×10 mol/L, respectively. In water phase, HA dissociates in the following way:
HA ⇌ H + A _K=1.0×10
And in benzene phase, HA conducts dimerization:
2HA ⇌ (HA)
(a) Calculate the equilibrium concentration of all of chemical species in water phase and the pH.
(b) Calculate the equilibrium constant (K) of dimerization of HA in benzene.
(c) There is a benzene ring in the structure of HA, and there are 3.85×10molecules in 1.00 g HA. Derive the name of HA.
(d) Explain why HA conducts dimerization in benzene by drawing the structure of the dimer.
Day - Feb 7, 2020
PhysC[SChO] Electrochemistry is an area in chemistry with wide applications. For every electro-chemical cell, we may determine unknowns (such as concentration or E_values) by making appropriate measurements. In this question, we will explore different electrochemical systems through a series of calculations.
We will start by determining the concentration of an unknown solution based on electrochemical measurements.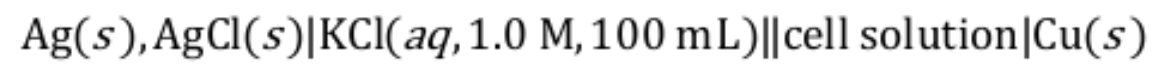
An electrochemical cell is constructed as shown above. The cell solution consists of:
(a) The measured voltage was -0.440 V. Calculate the molarity of KOH solution.
(b) CN is able to form highly stable complexes with Ag and Cu to increase the solubility of their salts. Describe the properties of the CN ligand that allow it to bind strongly with Ag and Cu ions.
Given that: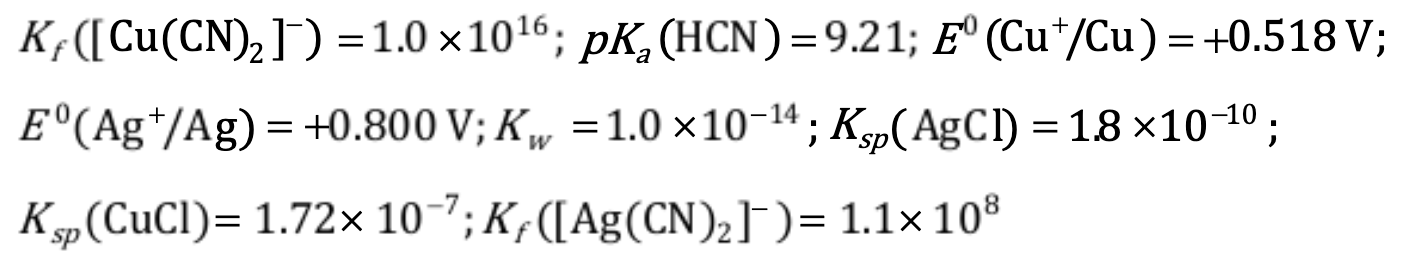
Electrochemistry may also be used to analyse the redox properties of a metal. Let us consider a theoretical metal M. A glass membrane M/M selective electrode gives rise to an equilibrium potential of -0.393 V against a Ag/AgCl reference of 0.222 V when placed in a 1 L standard solution of 0.100 mol/L M ion prepared by dissolving MCl salt in water. 5.00 g of NaHEDTA (_M_r = 336) is then added to the standard solution with a pH=12 buffer (all EDTA exists in Y form). A new cell potential is obtained at equilibrium.
(c) Calculate the standard reduction potential of M. If you are unable to obtain a value, please use -0.100 V for the following calculations.
(d) Calculate the final cell potential obtained.Given that: _K of [M-EDTA] complex = 22.3
M follows a theoretical Latimer diagram as shown below: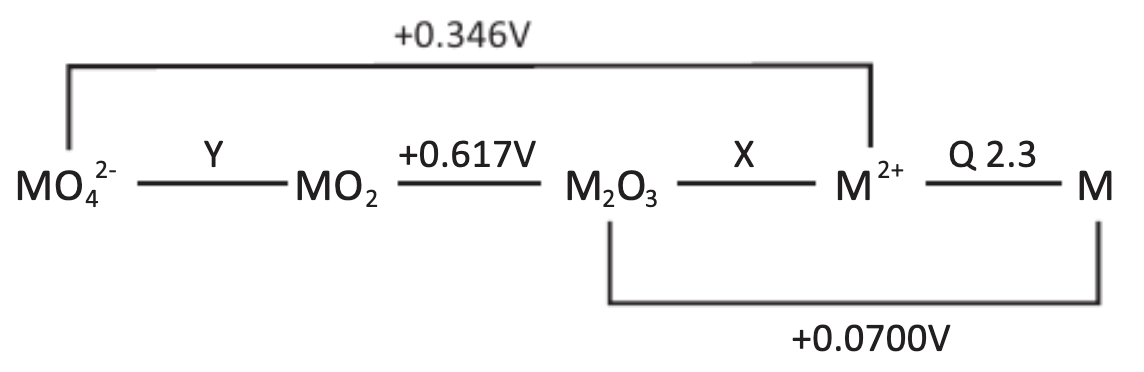
(e) Calculate the unknown reduction potentials X and Y.
(f) Plot a Frost-Ebsworth Diagram for M.
(g) Using the Frost-Ebsworth Diagram, state the most stable oxidation state of M.
(h) Using the Frost-Ebsworth Diagram, find out which M-containing species will tend to undergo disproportionation. Write the balanced disproportionation reaction with stoichiometric coefficients. Calculate the equilibrium constant for the disproportionation.
Day - Feb 5, 2020
AanalC[IChO1987-P3, revised] 25.00 cm of a neutral solution containing potassium chloride and potassium cyanide are potentiometrically titrated with a standard 0.1000 molar silver nitrate solution at 25 °C using a silver electrode and a normal calomel (HgCl(s)/Hg(l)) half-cell with KNO - salt bridge. The protonation of cyanide ions is negligible. The potentiometric curve obtained (emf (V)) vs. burette readings (in cm) is shown below.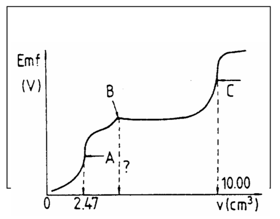
Prep Question:
i) What is the relationship between the emf with free [Ag] during the titration?
ii) Which one will precipitate first, AgCl or AgCN?
iii) Before the formation of the first precipitate, a complex is produced. What is the complex and why it is formed before the precipitation?
_
(a) The end points of the reactions taking place during the titration, are marked with A, B and C. Write the balanced ionic equation for each reaction.
(b) What volume of the titrant is required to reach point B?
(c) Calculate the concentrations of KCl and KCN (in mol dm) in the sample solution.
(d) Calculate the emf readings at the points A and C in volts.
(e) What is the molar ratio Cl/CN in the solution and in the precipitate at point C?

Update on Feb 7, 2020:
Key of the first part seems like incorrect.
β indicates that the complexation of Ag with CN occurs easily. Thus A denotes the point where all Ag is present in the complex form, having a higher potential than Ag, B shows the point where the precipitation of AgCN starts, thus leading to a constant Ag concentration until all CN is precipitated. Now at point C the precipitation of the more soluble AgCl begins:
A: Ag + 2 CN → [Ag(CN)]
B: [Ag(CN)2]- + Ag+ → 2 AgCN↓
C: Ag +Cl →AgCl↓
The proposed correction is as follows:
A denotes the point where all Ag is present in the complex form. AgCN starts to precipitate during A to B. B is the point where all CN- is preciated and the precipitation of AgCl starts, thus leading to a constant [Ag] = sqrt[Ksp(AgCN)] until most Cl is precipitated, which indicates the precipatation of AgCN during A to B. C denotes the point that all Cl is precipitated (equivalence point).
A: Ag + 2 CN → [Ag(CN)]
B: [Ag(CN)2]- + Ag+ → 2 AgCN↓
C: Ag +Cl →AgCl↓
Day - Feb 3, 2020
Data at 298 K:
pKa
Amines: MEAH+/MEA; MDEAH+/MDEA p_K_a = 9.5
CO(aq) p_K_a1 = 6.4; p_K_a2 = 10.3
HS p_K_a1 = 7.0; p_K_a2 = 13.0
CHSH p_K_a = 10.3
AnalC[IChOPrep2019-Q6]** 95% of dihydrogen is produced by steam reforming from natural gas. The corresponding reaction is analoguous to the reaction with methane (reaction (1)), which is carried out at about 900 °C in presence of a catalyst. (1)
35% to 40% of the dihydrogen thus obtained is used in ammonia synthesis. However, one sulfur atom per 1000 nickel atoms is sufficient to poison the nickel-based catalyst. Since acidic gases (HS and CO) contained in natural gas can also damage the pipelines, natural gas must be deacidified and desulfurized.
Steam reforming from natural gas
1. Give the chemical reaction of steam reforming for an alkane CnH_.
2. Calculate the equilibrium constant _K° of reaction (1) at 900 °C.
Removal of acidic gases
A common method to remove acidic gases from natural gas is to use an amine solution. Some amine solutions can solubilize all the acidic gases, whereas others are selective due to kinetic differences between HS and CO. This process is modeled below, replacing the hydrocarbons by N. The following experiments aim to study deacidification with two different amines: monoethanolamine (MEA) and methyl-diethanolamine (MDEA), using the apparatus depicted below.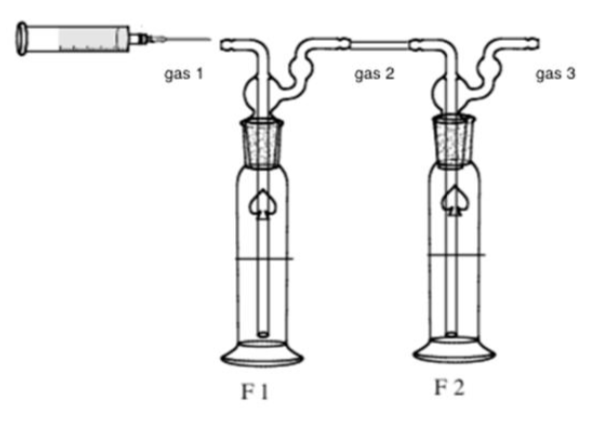
Flask F1 initially contains 100 mL of a 0.5 mol L amine solution (n = 50 mmol: large excess).
Flask F2 initially contains 100 mL of a 0.5 mol L NaOH solution (large excess too).
Step. 1: a gas sample (gas 1) is driven by N into a flask containing an amine solution; the outgoing gas (gas 2) bubbles in a second flask containing a NaOH solution; the final gas (gas 3) no longer contains acidic gas.
Step. 2: the liquid contents of each flask are titrated by an HCl solution (c = 1.0 mol L). Both pH and conductivity are recorded along the titration, so that two curves are obtained for each experiment (see below).
The sample of gas 1 contains n mmol of CO, n mmol of HS and n mmol of CHSH. The first experiment is carried out with the primary amine MEA; the second one with the tertiary amine MDEA.
3. Write down the thermodynamic quantitative (K° >> 1) reactions between the different gases and (i) the amine solution and (ii) the NaOH solution.
We first study the experiment with MEA. There is no kinetic blockage with this amine.
4. Determine the amount of each species in the solution (as a function of n, n and n) in the flask F1 before titration.
5. Which chemical species is/are present in gas 2?
6. Using the curves A1F1 and A1F2, determine (i) n and (ii) a relation between n and n.
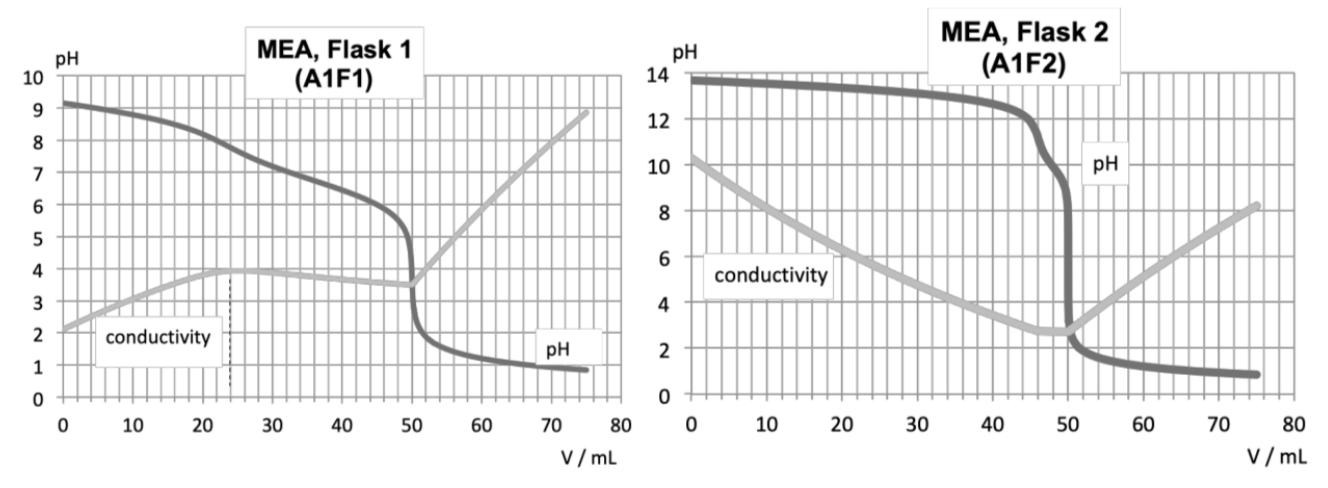
MDEA reacts with only one of the acid species, the other reaction being kinetically blocked.
7. Determine the amount of the reacting species using curve A2F1.
8. Using curve A2F2, determine if MDEA selectively reacts with CO or with HS. Calculate the two remaining n and n.
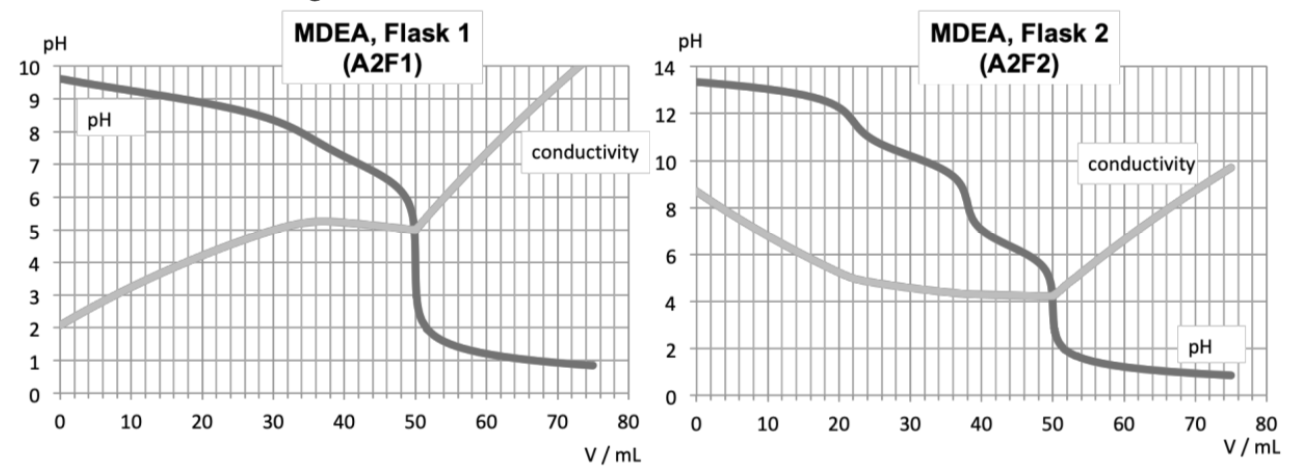
Update: Why the conductivity decreasses in the last figure when HCl is added to neutralized the OH-?
Day - Jan 31, 2020
AnalC[IChOPrep2012-Q11] Lead chromate has been widely used as a paint pigment, although this usage has been curtailed by envi- ronmental concerns in recent decades. Both components of this compound are hazardous to human health. Chromate is of particular concern because it is extremely mobile in groundwater. Therefore, humans can be exposed when they drink water from wells that are located at great distances from industrial sources of chromium.
The following equilibrium constants are given.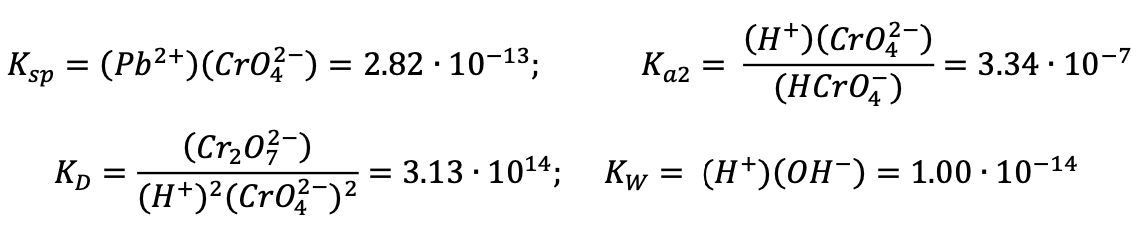
(a) Suppose that PbCrO(s) in a landfill dissolves to equilibrium in groundwater that has a pH of 6.00. Calculate the equilibrium concentrations of Pb, CrO, HCrO, and CrO.
(b) A toxicologist wishes to know at what total dissolved chromium concentration (Cr) the equilibrium concentration of HCrOis equal that of CrO in the human stomach. Assuming that the human stomach is a dilute solution with pH = 3.00, calculate Cr.
Day - Jan 29, 2020
Inorgo[BCC2018-Q3] The metal Me, when chopped into fine pieces, can be dissolved in a mixture of concentrated nitric and hydrofluoric acids, and the process yields gaseous productsA and C, ternary compound B (a white solid), and odorless, colorless liquid D. The density of A is 9.925 times that of C. C is the only nitrogen-containing compound. Compounds Aand B both contain Me in its maximum oxidation state, and hydrolysis of compounds A and B produce a yellow-colored solid E. Compound Eisn’t soluble to any appreciable extent in even mildly acidic solutions; however, when it is washed with a solution of NaOH, it dissolves to form compound F (13.936% Na, 55.735% Me by mass). When 1.000 g of compound B is mixed with excess barium acetate solution, 2.6676 g of white precipitate forms.
(a) What is metal Me?
(b) Write the molecular formula of D.
(c) Write the molecular formulas of gases A and C.
(d) Write the molecular formulas ofE and F.
(e) Write the molecular formula of B.
Day - Jan 27, 2020
Inorgo[NCChO2019-Q1] Figure 1-1 shows an interesting “tanghulu, candied gourd” molecule. A, B, C and D are located in adjacent periods of the same group, and the electronegativity of B is greater than that of D. C can form binary compounds with A, B, and D, respectively, and C and E can form volatile compound CE. CE can be used for bleaching and sterilization in industry. A and E can form the compound AE (bp, 76 C). X is one of the complete hydrolysis products of AE, a common reducing agent for metal plating of plastic parts. A, C, and E can form a six-membered ring compound A**C**E. In chloroform, D and E can bind with two methyl groups to form the compound DEMe.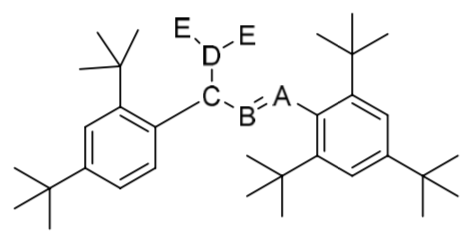
Figure 1-1
1-1 Write down the elements represented by A, B, C, D, and E.
1-2 Write down the hybrid orbitals of A and C in the molecule of “tanghulu”, respectively.
1-3 Draw the structural diagram of the molecule made by A with its highest oxidation state and element E, and specify the symmetry element (such as C) of the molecule.
1-4 Draw all possible stereoisomers of DEMe.
1-5 Indicates that X is a monoprotic, diprotic, and triprotic acid, and write the reaction equation of nickel sulfate reduced by X in a weak acidic medium.
Day - Jan 24, 2020
Orgo[MIChO2008-Q6] In the 80s of the 20th century, the reactive compound X was synthesized. The precursor of X - substance D - is obtained in four steps, starting from tetranitromethane [C(NO)]. The interaction of C(NO) with KOH leads to A and K. Substance A, upon addition of HSO (conc.), is converted to compound B, which further reacts with methyl vinyl ketone, gives C with a composition of CHNO. Destructive nitration of C with excess mixture of concentrated nitric and sulfuric acids gives D, which contains carbon atoms with the same environment, doesnot contain hydrogen atoms and has a molar mass of 300 g/mol. Vacuum pyrolysis of D (270 °C, 1 mmHg) results in X sublimated in the form of greenish-yellow crystals and the evolution of a reddish brown gas E, which condenses into a colorless liquid F upon cooling. The reaction of X with anthracene results in a symmetrical compound G, which, when heated in vacuum, cleaves into E and yellow crystals of H. The reaction of cyclopentadiene with H gives isomeric products I and J.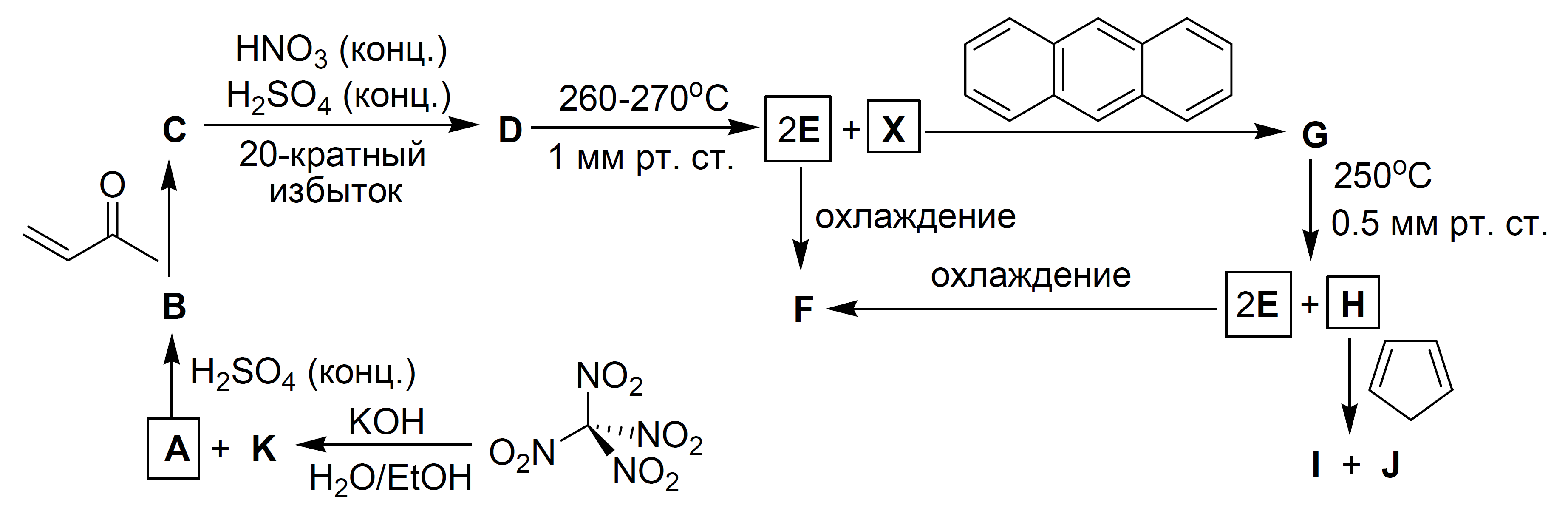
конц. = conc.; 20-кратный избыток = 20x excess; 1 мм рт. ст. = 1 mmHg; охлаждение = cooling;
1. Decipher the given scheme. Draw structural formulas of A-J and X.
2. Suggest a method for producing K from E.
3. Arrange the stability of conjugate bases of nitromethane, dinitromethane and trinitromethane.
4. Write the equation for acid-base equilibrium of nitromethane in water and calculate the pH of a 1 M nitromethane solution if the pK of nitromethane is 10.2. Give the resonance structures of the nitromethane anion.
Day - Jan 22, 2020
AnalC[NCChO2015-Q2] The silver electrode was put into a mixed solution of 1.000×10 mol∙L NHNO and 1.000×10 mol∙L AgNO at 298 K, and its electrode potential φ(Ag/Ag) was measured as a function of pH of the solution. As shown below. It is known that the dissociation constant K of ammonia is 1.780×10, the ideal gas constant R = 8.314 J∙mol·K, and the Faraday constant F = 96500 C∙mol.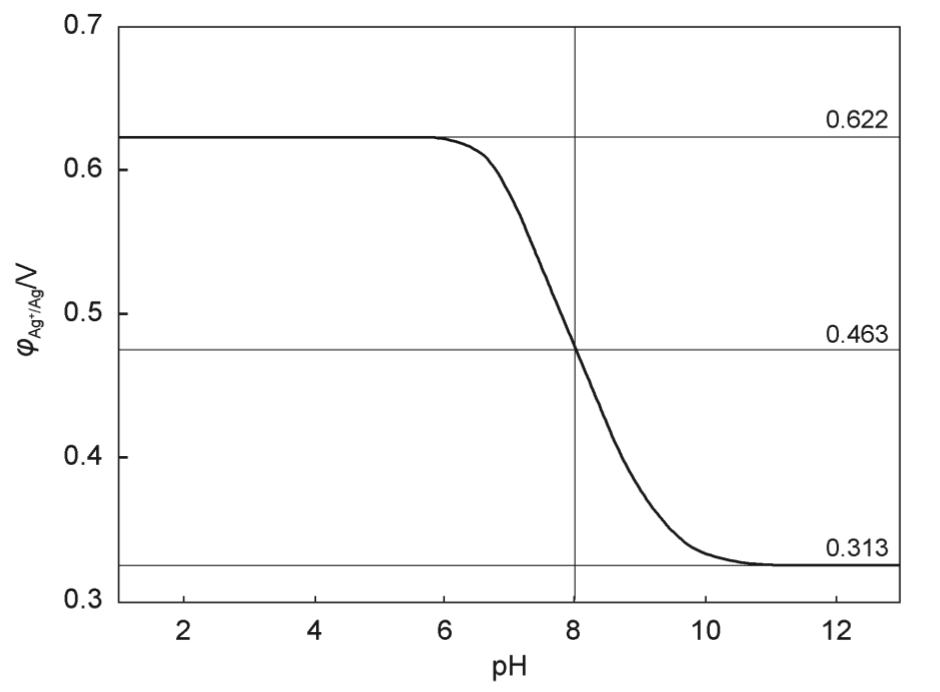
1 Calculate the standard electrode potential φ of the silver electrode in 298 K.
2 Calculate the stepwise stability constants K and K of the silver-ammonia complex ion.
3 Design a galvanic cell using the coordination reaction of silver ions with ammonia. The battery reaction equation is:
Calculate the standard electromotive force of the galvanic cell. If the standard stability constants of the first and second step of the silver-ammonia complex ion cannot be calculated, it can be assumed that they are both 1.00×10.
Day - Jan 20, 2020
AnalC[MIChO2007-Q2, revised] 10.2 g colorless crystalline substance A was dissolved in 1000 mL of water. An aliquot of the resulting solution of 10.0 mL was transferred to a titration flask containing a known and excess of KBrO and KBr in a 1:5 molar ratio. After the completion of the redox reaction, the resulting mixture was buffered and then titrated with sodium formate solution, and 7.5 mL of a 0.050 M titrant solution was consumed. Another aliquot of solution A of 10.0 mL was transferred into the electrochemical cell and subjected to coulometric titration with electrogenerated OH ions. Moreover, only one jump is observed on the titration curve.
1. Determine substance A, if it is known that the substance loses 6.6% of the initial mass with the formation of substance A1 when heated above 200 °C, and then loses 31.5% of the mass of obtained A1 when further heated above 600 °C. Write the equations of reactions underlying the titrimetric determination of substance A, as well as its thermal transformations.
2. Determine the amount of electricity [unit: C] that went into titration of substance A, if the current efficiency is 90% (Faraday constant F = 96500 C/mol).
3. Determine the K of acidic group in compound A, if it is known that the pH of 0.10 M A solution is 1.54.
4. The standard gravimetric method for determining the concentration of A is to convert A into a white crystalline precipitate B with a solubility product K = 1.3·10. Identify compound B if the molar mass of B is about 1.7 times of that of A.
5. Calculate the solubility of substance B in pure water (mg/L).

