- Problem 1. Salvia Species Growing in Turkey: Isolation and Total Synthesis of Abietane Diterpenoids
- Key to Problem 1 - by Young
- Problem 2. Istanbulins and RelatedSesquiterpene Natural Products
- Key to Problem 2
- Problem 3. Çay, Cha, Chai, Te, Tea,Tee, Thé, Thee, and Earl Grey Tea Flavor: Bergamot
- Key to Problem 3
- Problem 4. Early Russian Organic Chemistsand Markovnikov’s Rule
- Problem 5. Arndt–Eistert Homologation
- Key to Problem 5 - by Alec
- Problem 6. Atovaquone
- Key to Problem 6 - by Alec
- Problem 7. Which is (±)-Trikentrin A?
- Key to Problem 7 -by Alec
- Problem 8. Stereoisomers of 1,2,3-Triphenylpropane-1,3-diol
- Problem 9. NMR, Symmetry, and Structural Analysis
- Problem 10. Woodward–Hoffmann Rules and Pericyclic Reactions
- Problem 11. Benzoporphyrin
- Problem 12. Blue to Green, Turquoise
- Key to Problem 12 by Dr. Chen
- Problem 13.
- Problem 14.
- Problem 15.
- Problem 16.
- Problem 17.
- Problem 18.
- Problem 19.
- Problem 20.
(version 1, published on January 31, 2020)
keys are provided by Young, Alec, and Dr. Chen, thanks for their contributions.
https://icho2020.tubitak.gov.tr/icho-2020-hazirlik-sorular%C4%B1
Problem 1. Salvia Species Growing in Turkey: Isolation and Total Synthesis of Abietane Diterpenoids
The genus Salvia, named after a
Latin word, salvare (“healer”), has a variety of species with important
medicinal activities. They have been used for
the treatment of colds, flu, and menstrual disorders in most regions of the
world since ancient times. In
Turkish folk medicine, Salvia L.
species have also been used as a carminative, diuretic, hemostatic,
spasmolitic, and stomachic, and in the treatment of mouth and throat irritations
due to their antibacterial and wound healing properties. The genus Salvia includes over 900 species across
the world, 58 of which are endemic in Turkey.
Female Turkish scientists Ulubelen & Topçu
with co-workers have studied Anatolian _Salvia
_plants growing in Turkey, and isolated and characterized more than 320
natural products, most of which are terpenoids, while one third are new
diterpenoids.

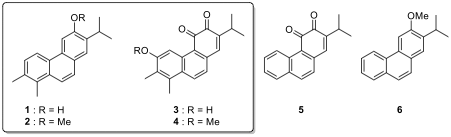
In one of their studies on Salvia multicaulis Vahl., Ulubelen & Topçu isolated four new aromatic abietane norditerpenoids (1–4), which showed strong antituberculous activity. In addition to the antibacterial and antifungal activities of the isolated diterpenoids, the plant extracts also showed antioxidant, antiinflammatory, and cholinesterase inhibitory activities. _S. multicaulis _has folkloric use in Anatolia, such as an appetizer, for wound healing, against scorpion stings, and in the treatment of respiratory and urinary infections and diabetes.
Later, a research group in Turkey developed a
synthetic route to obtain derivatives of natural products 1–4. This problem covers
the synthesis of related compounds. The following reaction schemes illustrate the total
synthesis of diterpenoids 1 and 5.
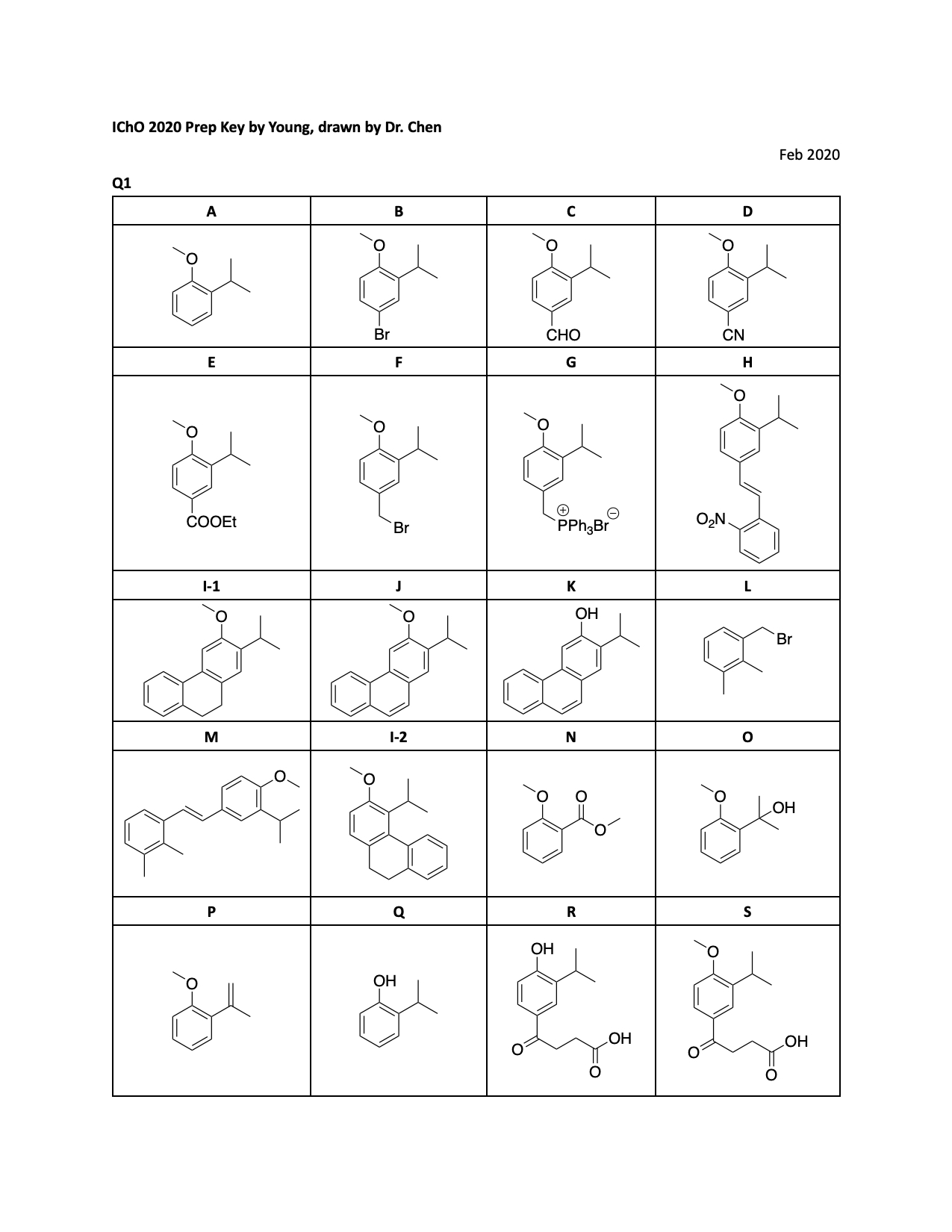
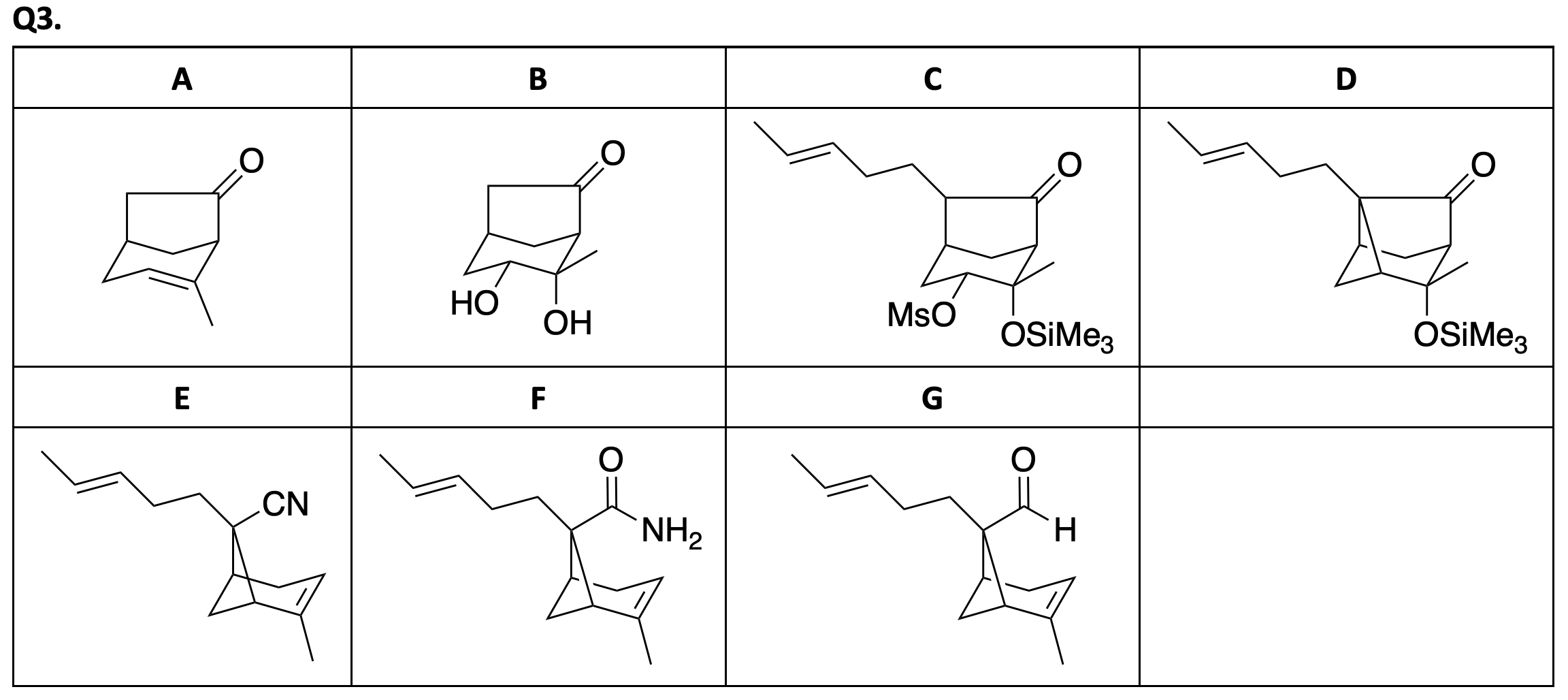
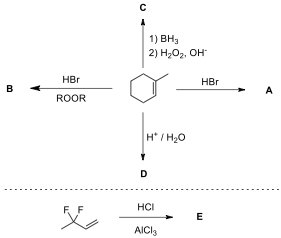
1.1. Draw the structure of the products A–M,
without any stereochemical detail. Hint: In second step
, combination of lithium bromide and cerium(IV)
ammonium nitrate (CAN) is used as a brominating reagent. Compound C is a benzaldehyde derivative and used
in the synthesis step of compound M.
1.2. During the cyclization of H to I-1, another isomeric compound,
I-2, with the formula CHO,
is also formed. Draw the structure of I-2.

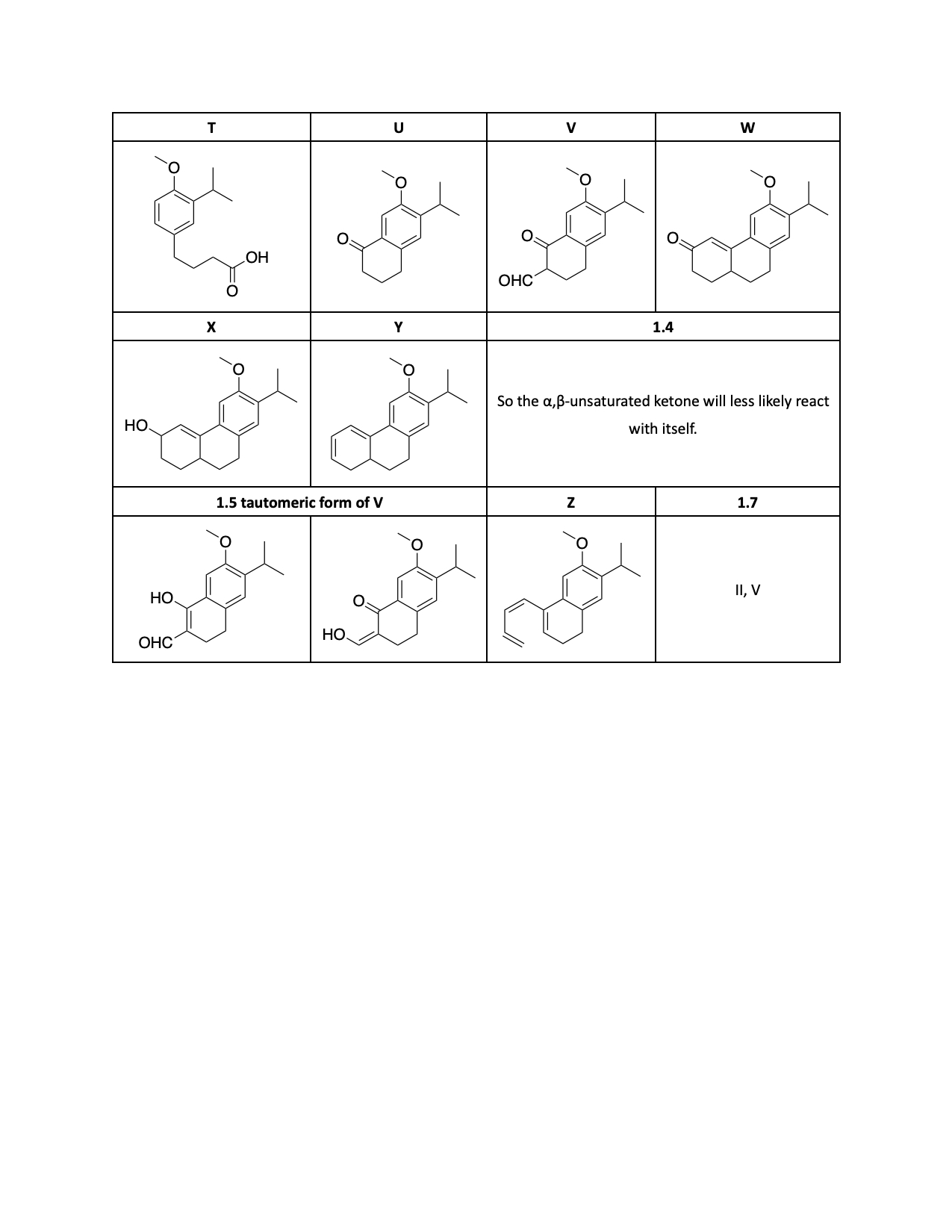
1.3. The following reaction scheme is related to the synthesis of 6, a desmethyl derivative of the diterpenoids 1 and 2. Draw the structures of products N–Y, without any stereochemical detail. Hint: Compounds R, S and T exhibit acidic character. The transformation of compound V to W includes Robinson annulation and a possible deformylation reaction steps.
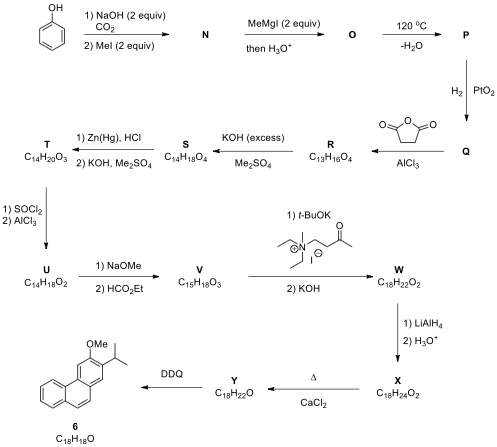
1.4. During the transformation of compound V to W (Robinson annulation
step), the use of a precursor of the α,β-unsaturated ketone, such
as a β-chloroketone or N,N,N,-trialkyl-3-oxobutan-1-aminium
halide (as used in the reaction scheme), can be more favorable. Explain.
1.5. Draw possible tautomeric forms of compound V.
1.6. Compound Y can be also obtained via ring-closing
(electrocyclization) of the compound Z. Draw structure of Z.
1.7. For the transformation of X to Y, which of the following
reagents can also be used? (Ignore S2’ type reactions).
| ☐ | i) PBr/pyridine; ii) n-BuSnH/AIBN |
|---|---|
| ☐ | i) PBr/pyridine; ii) Na/t-BuOH |
| ☐ | i) MnO; ii) DDQ |
| ☐ | i) TsCl/pyridine; ii) LiAlH |
| ☐ | i) TsCl/pyridine; ii) DBU |

Key to Problem 1 - by Young
Problem 2. Istanbulins and RelatedSesquiterpene Natural Products

Some elements received their names from
different places around the world. In this respect, the record belongs to the
Swedish village of Ytterby, after which four elements were named: ytterbium
(Yb), yttrium (Y), erbium (Er), and terbium (Tb). However, elements are not the
only chemical entities that owe their names to such places. Interestingly, a
class of natural products, istanbulins A–E,
received their names from the city of Istanbul. The first two members of this
family, istanbulins A and B, were first isolated by Prof. Dr. Ayhan Ulubelen
and co-workers from the plant Smyrnium
olusatrum in 1971. The isolation of the remaining members, istanbulins C–E,
was reported by Ulubelen and co-workers between 1979 and 1982.
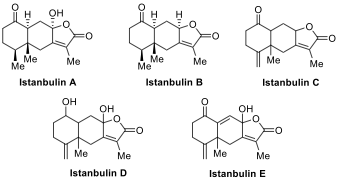
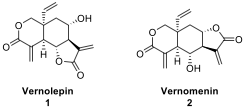
Istanbulins constitute a subclass of a much
larger family of natural products called sesquiterpenes. Two important
sesquiterpene natural products with a similar 6-6-5 fused ring system are
vernolepin (1) and vernomenin (2). Danishefsky and co-workers reported
an elegant total synthesis of these two natural products in 1976 via the
utilization of the Diels–Alder (DA) chemistry of the so-called Danishefsky’s
diene.
Please note that all formulae depicting chiral
molecules in this question refer to racemic mixtures.
In this context, Danishefsky’s diene (3) and the Rawal–Kozmin diene (4) are two electron-rich dienes that
found widespread use in organic synthesis, and their structures are shown
below.
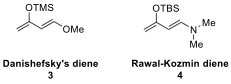
TMS: trimethylsilyl; TBS: tert-butyldimethylsilyl
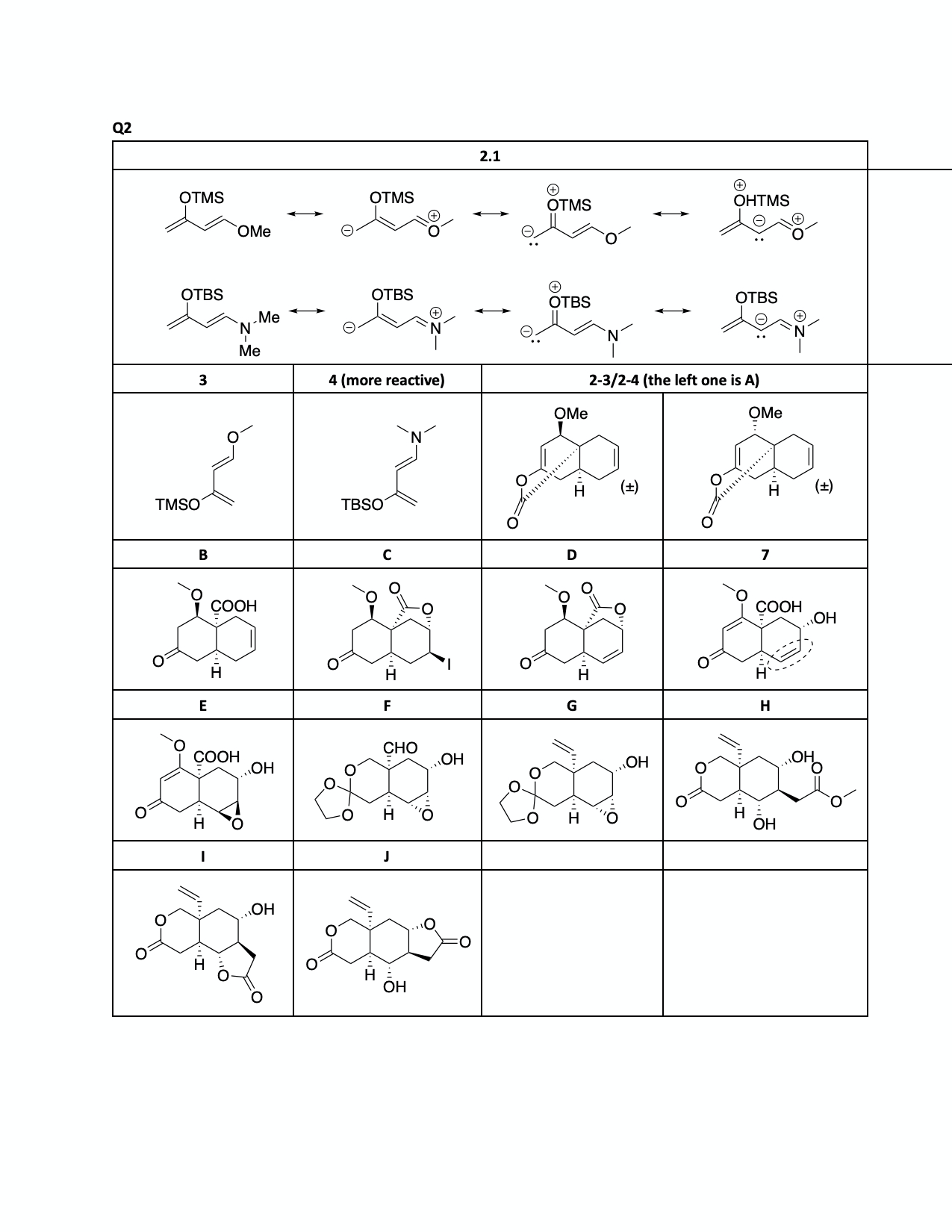
2.1. Draw the major resonance structures of dienes 3 and 4. Indicate the
carbon atoms with higher electron density on each diene.
2.2. Compounds 3
and 4 have been extensively used as
diene components in Diels–Alder reactions. Draw
the conformations of 3 and 4 required to be able to enter a DA
reaction. Predict which
compound is a more reactive diene in a DA reaction with maleic anhydride (5).
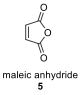
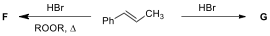
2.3. When a mixture of Danishefsky’s diene (3) and compound 6 was heated followed by treatment with acid (TsOH, p-toluenesulfonic acid), compound A was obtained as the major product.
Draw the structures of all possible Diels–Alder products with the molecular formula of CHO that can be obtained from the reaction of 3 and 6. Drawing only one enantiomer of an enantiomeric pair is sufficient.
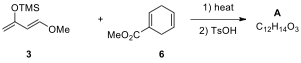
2.4. Determine
the structure of the major product A.
2.5. Diels–Alder adduct A was converted to compound 7
via a sequence of 4 steps as shown below. Compound B is known to be acidic. Draw
the structures of B–D.
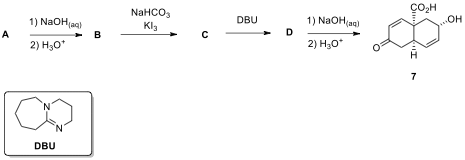
2.6. When compound 7 is reacted with 1 equiv of m-CPBA,
product E was obtained as a major
product. Circle the
functional group that reacts selectively with m-CPBA, and draw
the structure of E.
2.7. The syntheses of vernolepin (1) and vernomenin (2) were completed as shown in the scheme below. Draw the structures of compounds F–J. In the final step, compound I is the precursor of 1.
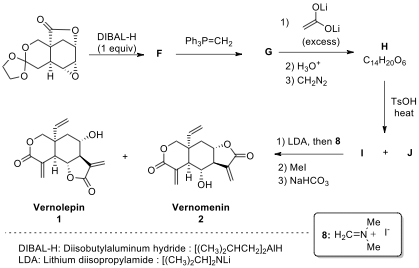
Key to Problem 2
Problem 3. Çay, Cha, Chai, Te, Tea,Tee, Thé, Thee, and Earl Grey Tea Flavor: Bergamot
| Cha | Chinese, Japanese, Korean, Portuguese….. |
 |
| —- | —- | :—-: |
| Chai | Russian, Persian… | |
| Çay | Turkish, Azerbaijani… | |
| čaj | Bosnian, Croatian, Czech, Serbian, Slovak.. | |
| Shay | Arabic… | |
| Te | Italian, Spanish… | |
| Tea | English… | |
| Tee | German… | |
| Thé | French… | |
| Thee | Dutch… | |
| Chaay | Hindi… | |
| ….. | … | |
|
| —- | —- | :—-: |
| Chai | Russian, Persian… | |
| Çay | Turkish, Azerbaijani… | |
| čaj | Bosnian, Croatian, Czech, Serbian, Slovak.. | |
| Shay | Arabic… | |
| Te | Italian, Spanish… | |
| Tea | English… | |
| Tee | German… | |
| Thé | French… | |
| Thee | Dutch… | |
| Chaay | Hindi… | |
| ….. | … | |

Tea (in Turkish: çay) is popular throughout Turkey and the Turkish diaspora. Turkish tea culture also extends from Azerbaijan to some countries in the Balkan Peninsula. Turkey has the highest per capita tea consumption in the world, i.e. 2.5 kg/person per year, followed by the United Kingdom (2.1 kg/person per year).


Bergamotene and derivatives (1-4), sesquiterpenes, are analogues of pinnae monoterpenes. Found
in bergamot oil, the bergamotenes contribute to the aroma and flavor of Earl
Grey tea. 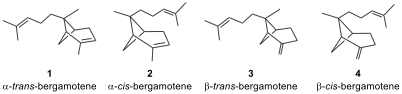
3.1. The following reaction scheme illustrates the synthesis of α-trans-bergamotene
(1). Draw the structures of products A~G.
3.2. What is the function of MeNO
reagent in the transformation of A
to B?
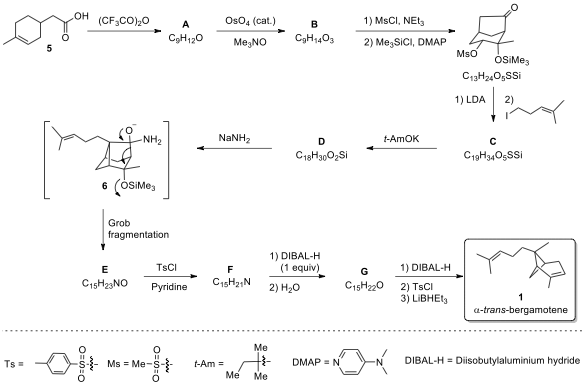
Key to Problem 3
Problem 4. Early Russian Organic Chemistsand Markovnikov’s Rule

The last year was devoted to the 150 anniversary of the
discovery of Markovnikov’s rule, formulated by Vladimir V. Markovnikov in 1869.
Markovnikov was a PhD student of the famous early Russian scientist Alexander
Butlerov. In his PhD thesis in 1869, Markovnikov discovered the famous rule
that exists in almost every textbook on organic chemistry. According to
Markovnikov’s rule, when an unsymmetrical alkene or alkyne reacts with a
hydrogen halide (hydrogen chloride, hydrogen bromide, or hydrogen iodide), the
hydrogen atom of HX adds to the carbon atom having the highest number of
hydrogen atoms. However, depending on the reagent or substrate, in some cases,
opposite results could also be possible, and these kinds of reactions are
called anti-_Markovnikov addition. Although Markovnikov’s rule was
developed for and is specifically applied to the addition of hydrogen halides
to alkenes or alkynes, many other additions are also described as Markovnikov
or _anti-_Markovnikov depending on the regioselectivity of the addition
reaction.
Actually, the rule should be revised as follows: “_addition to this
kind of double or triple bond proceeds through more stable intermediates”.
In some cases, besides electronic effects, steric effects can also affect the
formation of Markovnikov or anti-Markovnikov addition products.
The following problems are mainly related to discoveries described by
the student of the more distinguished
organic chemist Alexander Butlerov or his colleagues at Kazan University,
Tatarstan, Russia.
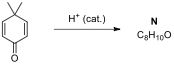
4.1. Draw the structures of major products A-E, including the appropriate
stereochemistry (ignore optical isomerism).
4.2. Draw the structures of major products F
and G for the following reactions.
Wagner–Meerwein Rearrangement (WMR)
Wagner is another
famous scientist who worked at Kazan University contemporaneously with Butlerov
and Markovnikov. Wagner proposed that bornyl chloride undergoes an internal
rearrangement to form pinene. Meerwein then generalized this type of
rearrangement. Thus, this kind of reaction was named Wagner–Meerwein
rearrangement. These reactions take place when a carbocation is formed.
Generally, a carbocation is rearranged to a more stable carbocation, if
possible, by neighboring group migration. In addition, if the reaction does not
proceed through a carbocation or borderline carbocation intermediates,
rearrangements do not take place.
4.3. Considering the formation of intermediates for every reaction, draw the structures of reagents H and I and major products J–M.
Acid-catalyzed
Wagner–Meerwein Rearrangement
The acid-catalyzed
reaction of 4,4-dimethylcyclohexa-2,5-dien-1-one
resulted in the formation of a compound, the NMR data of which are given below.
For N; H NMR (300 MHz, CDCl): δ = 6.95
(d, J = 8.0 Hz, 1H), 6.61 (d, J = 2.8 Hz, 1H), 6.57 (dd, J
= 8.0, 2.8 Hz, 1H), 5.39 (bs, 1H), 2.16 (s, 3H), 2.14 (s, 3H). C
NMR (100 MHz, CDCl): δ 153.4, 137.9, 130.4, 128.6, 116.6, 112.3,
19.8, 18.7.
4.4. Find the structure of product N and propose a
plausible mechanism.
4.5. What
kind of difference do you expect in the H NMR spectrum after a drop of DO is added to the solution in the NMR tube?
Zaitsev’s Rule
Zaitsev, who described a rule named after him (Zaitsev’s or Saytzeff’s
or Saytzev’s rule), was another PhD student of Butlerov’s. Zaitsev’s rule is an
empirical rule for estimating preferred alkene product(s) in elimination
reactions. At Kazan University, the chemist Alexander Zaitsev studied various
elimination reactions and observed a general trend in the resulting alkenes. More generally, Zaitsev’s rule stipulates that in
an elimination
reaction the most substituted product will be formed. The
following problem is mainly related to Zaitsev’s rule.
4.6. Draw the structures of elimination products O–Q and compound R. What is the major product formed by
the thermal reaction of R described
in the following scheme?
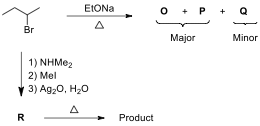
4.7._ _Which
base(s) can be used to increase the ratio of
Q relative to EtONa?
| ☐ | NaOMe |
|---|---|
| ☐ | KOMe |
| ☐ | _i-_PrOK |
| ☐ | _t-_BuOK |
| ☐ | NH |
| ☐ | DBU |
| ☐ | _i-_PrNEt |
Problem 5. Arndt–Eistert Homologation
Fritz Georg Arndt (6 July
1885–8 December 1969) was a German chemist who had a great influence on the
development of chemistry in Turkey. He was employed for two decades of his
professional life at Istanbul University in two distinct periods. He discovered
the Arndt–Eistert synthesis with Bernd Eistert. The Arndt–Eistert synthesis is
the chemical reaction for one-carbon homologation (i.e. the conversion of RCOH
to RCHCOH) of carboxylic acids and is called the
homologation process. In the Arndt–Eistert homologation, the key step is the
Wolff rearrangement of diazoketones to ketenes, which can be achieved
thermally, photochemically, or by silver (I) catalysis. The reaction is
conducted in the presence of nucleophiles such as water, alcohols, or amines to
capture the ketene intermediate to yield carboxylic acids, esters, or amides,
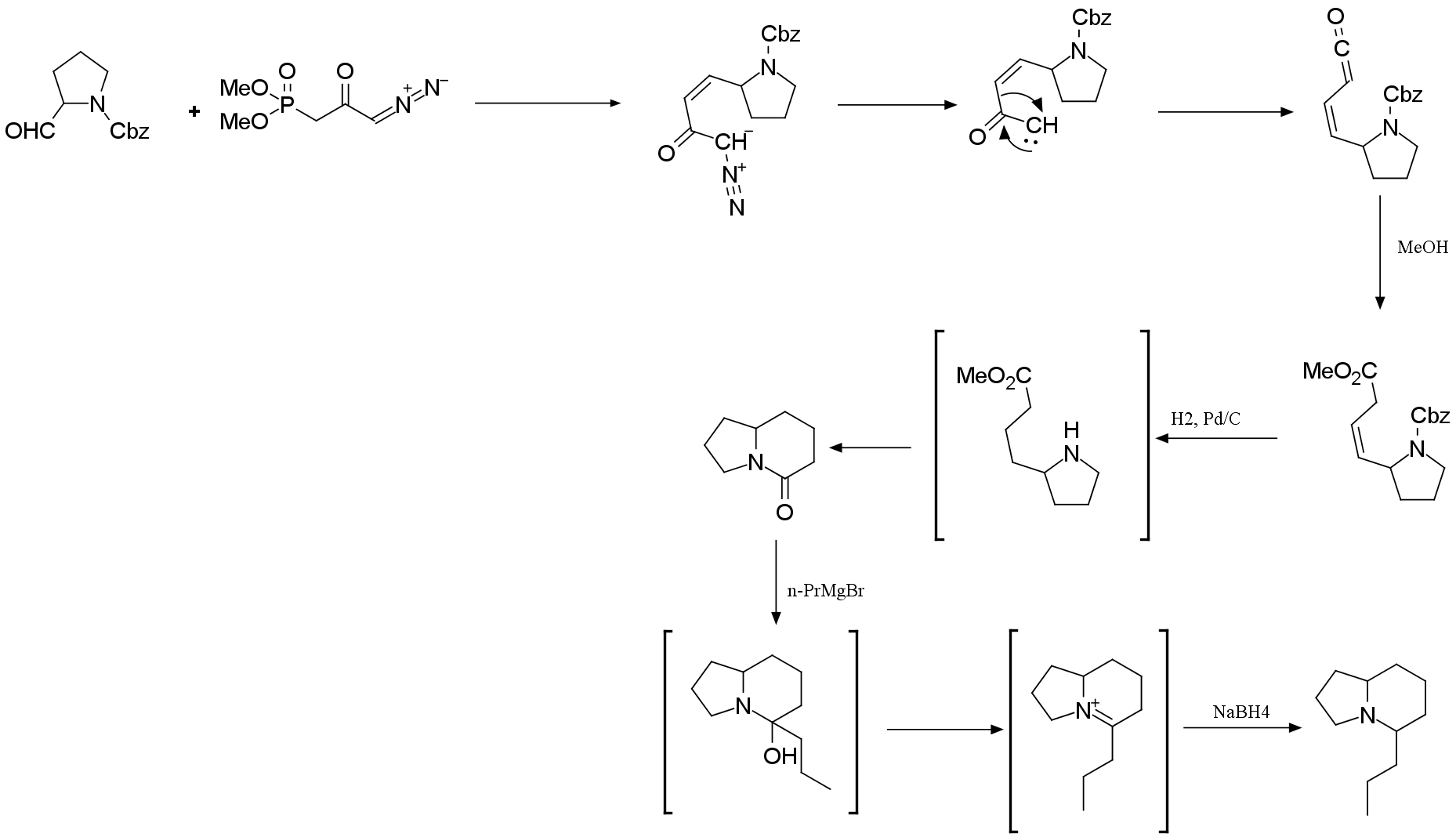
respectively. In this problem, synthesis of indolizidine alkaloids is studied.

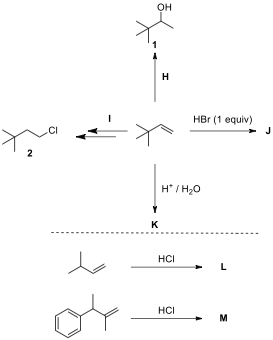
5.1. As depicted in the scheme below, the synthesis
of indolizidines 167B and coniceine could be easily and concisely achieved from β,γ-unsaturated ester B. The key step (A → B) is the Wolff rearrangement.
Compound C has a lactam core, which
is a bicyclic heterocycle containing a six-membered ring fused to a saturated
five-membered ring, one of the bridging atoms being nitrogen.
Draw the structures of A–D without any stereochemical detail.
5.2. In the Arndt–Eistert homologation reaction, an
α-diazo ketone can undergo photochemical Wolff rearrangement to form
α-ketocarbene via nitrogen extrusion. This intermediate undergoes a 1,2-alkyl
shift to give the ketene product.
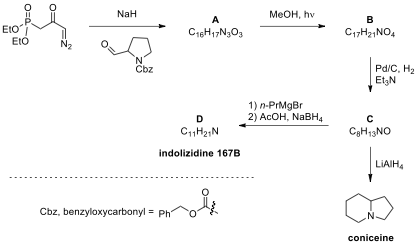
Draw the structures of the α-ketocarbene and ketene
intermediates in the second step (A → B).
5.3. Addition of propylmagnesium bromide to compound C, followed by AcOH/NaBH, is the last step in the total
synthesis of indolizidine 167B.
Draw the structure of an intermediate (CHN)
in the fourth step (C → D).
5.4. An alternative synthesis of coniceine is depicted below. Draw the structures of E–J.
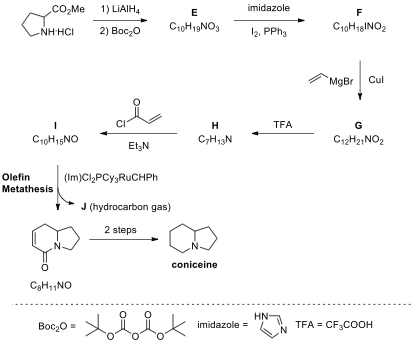
Key to Problem 5 - by Alec
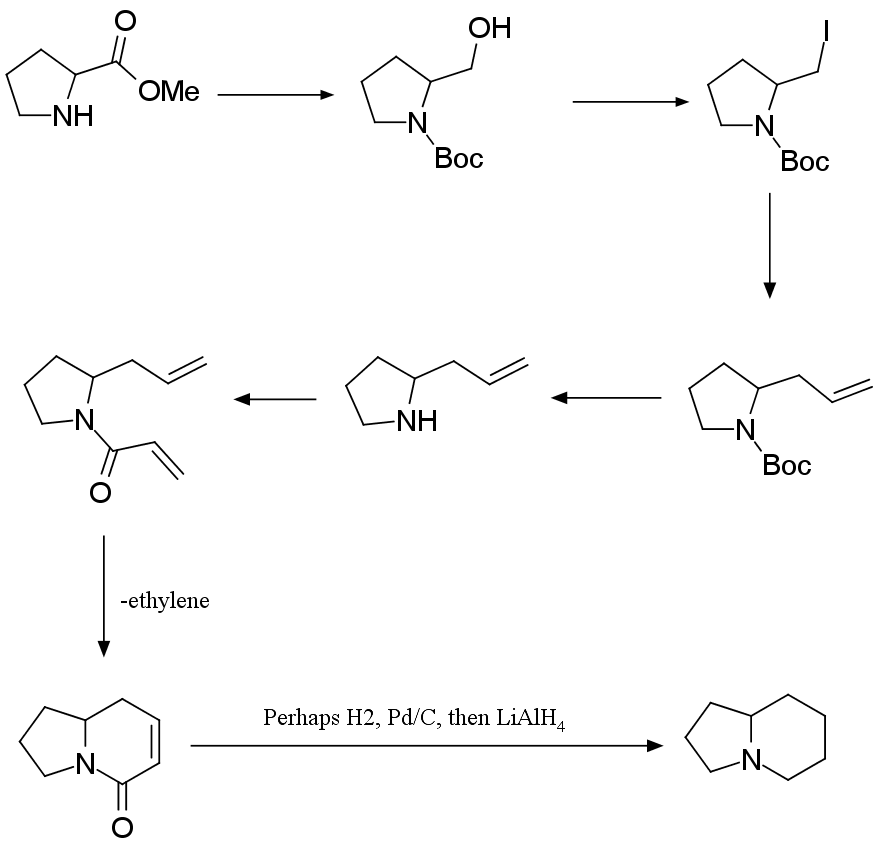
Problem 6. Atovaquone
Atovaquone, an approved drug, is used to treat pneumocystosis and malaria. Ketoester 1 and aldehyde 2 are key compounds in the synthetic process of atovaquone.
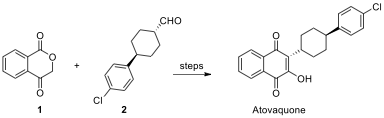
6.1. The synthesis of key compound ketoester 1 is shown below. A mixture of phthalic
anhydride and EtN is treated with diacid. Gas evolution is observed
during this period. Treatment of the reaction mixture with aq. HCl solution provides formation of acid 3 through intermediate A
with two carboxylic acid groups.
Acid 3 is converted to the isomeric
intermediate B, containing both
hemiacetal and ester functionalities, followed by dehydration to the alkene C, which is then brominated to give D under acidic condition. Dibromide D undergoes solvolysis in a hot mixture of HO/AcOH to
give tertiary carbocation intermediate E,
which is then trapped with water to give intermediate hemiacetal F. Finally, rearrangement of
intermediate hemiacetal F provides
key compound 1.
Note: The square brackets
denote that the product was not isolated but reacted further without
purification. The conversion of 3 to
1 is a one-pot reaction that
involves a series of reactions occurring one after another in the same vessel
without isolation and purification of intermediates.
_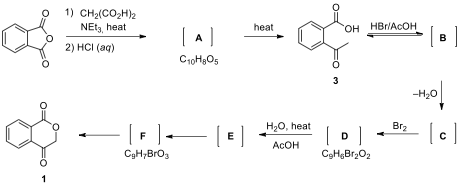
Spectroscopic data for intermediates B and C: B: H NMR = 7.86–7.52 (4H), 4.13 (bs, 1H, exchangeable with DO), 1.97 (s, 3H). C: H NMR = 7.92–7.58 (4H), 5.24 (m, 2H); C NMR = 166.8, 151.8, 139.0, 134.4, 130.4, 125.3, 125.1, 120.6, 91.3; MS m/z = 146.0
Draw the structure of intermediates A–F in the synthesis of 1.
6.2. The synthesis of aldehyde 2 starts from cyclohexene by key steps including Friedel–Crafts
acylations, haloform, reduction, and oxidation. Friedel–Crafts acylation of
cyclohexene with acetyl chloride yields chlorocyclohexyl methyl ketone J. Reaction of cyclohexene with acetyl
chloride produces an initial carbocation G
that undergoes two successive Wagner–Meerwein hydride migrations to form isomeric carbocations H and I, respectively.
Trapping of carbocation I with
chloride ion produces J, the
Friedel–Crafts reaction of which with chlorobenzene provides K. Haloform reaction of methyl ketone K using sodium hypochlorite (NaOCl)
gives the corresponding acid L. Acid
L is converted into the aldehyde 2 in a several-step reaction sequence.
Draw structure of isomeric carbocations G**I formed in this reaction.
6.3.** Are these carbocations chiral?
| G | ☐ | Yes |
|---|---|---|
| ☐ | No | |
| H | ☐ | Yes |
| ☐ | No | |
| I | ☐ | Yes |
| ☐ | No |
6.4. Draw the
structure of JL.
6.5. Choose
all correct statements for L.
| ☐ | L has 4 stereoisomers. |
|---|---|
| ☐ | L is a chiral compound. |
| ☐ | L is an achiral compound. |
| ☐ | L is a meso compound. |
| ☐ | L has 2 stereoisomers. |
| ☐ | Stereoisomers of L are diastereomers of each other. |
| ☐ | Stereoisomers of L are enantiomers of each other. |
6.6. Which of the following compound(s) result(s)
in the haloform reaction of K?
| ☐ | CHCl |
|---|---|
| ☐ | CHCl |
| ☐ | CHCl |
| ☐ | CCl |
6.7. Which of the following reagents are
appropriate to form aldehyde 2 from L?
Choose all correct reactions.
| ☐ | 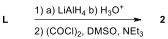 |
|---|---|
| ☐ | 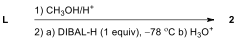 |
| ☐ | 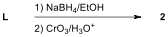 |
| ☐ | 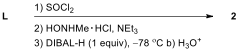 |
| ☐ | 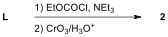 |
| ☐ | 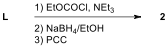 |
| ☐ |  |
Key to Problem 6 - by Alec
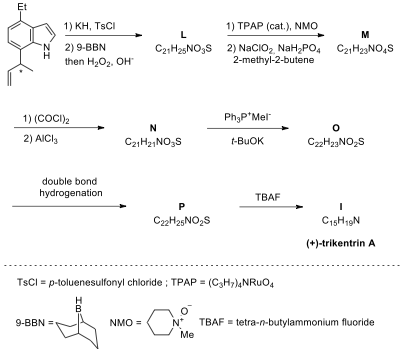
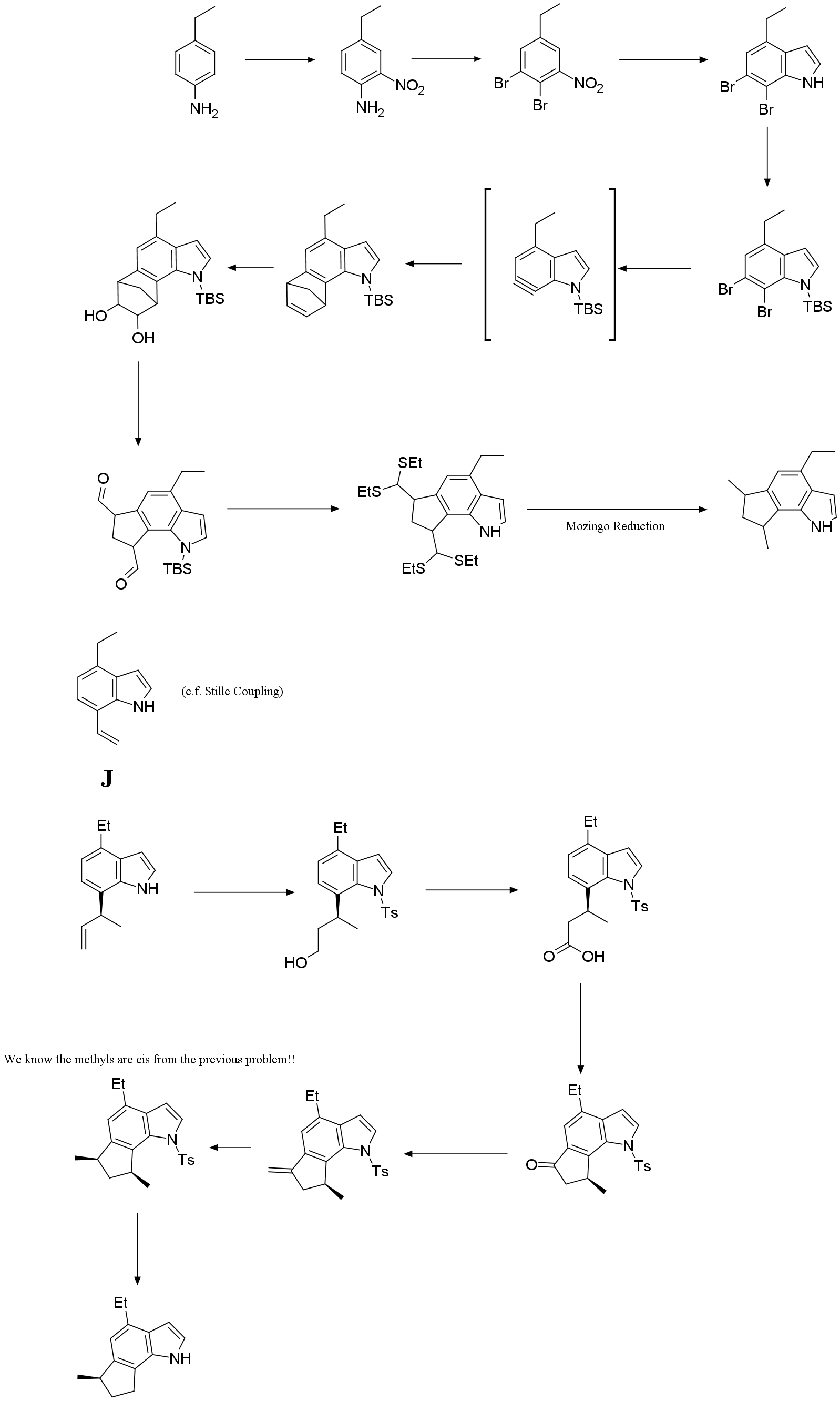
Problem 7. Which is (±)-Trikentrin A?
Although the indole skeleton is ubiquitous in nature, annulated indoles at any of the benzenoid positions are uncommon. The trikentrins and the structurally similar herbindoles represent fascinating such examples of 6,7-annulated indole or polyalkylated cyclopent[g]indole natural products. The trikentrins were isolated from the marine sponge Trikentrion flabelliforme and possess antibacterial activity. Possible structures for trikentrin A are shown in the Figure below. In this problem, we will find out which of these structures is trikentrin A.
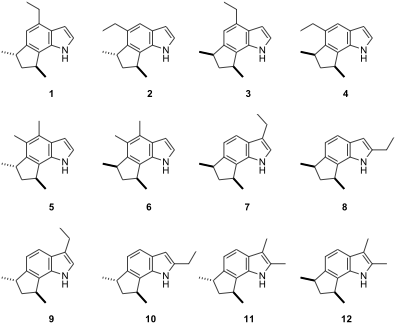
There are several ways to synthesize trikentrin
A. Two routes below involve aryne-based and hydrovinylation strategies and both
finally lead to the formation of trikentrin A. The first step for problems 8.1
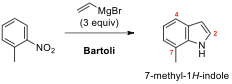
and 8.2 is the Bartoli reaction or Bartoli indole synthesis, which is the
organic reaction of ortho-substituted
nitroarenes with vinyl Grignard reagents to yield substituted indoles. In
particular, it is the most efficient route to 7-substituted indoles.
(±)-Trikentrin A: H NMR (CDCl): δ 8.08
(bs, NH, 1H), 7.156.59 (3H), 3.44 (dt, J = 8.8, 7.5 Hz, 1H), 3.22 (dt, J
= 8.8, 7.5 Hz, 1H), 2.94 (dq, J =
15.0, 7.5 Hz, 1H), 2.93 (dq, J =
15.0, 7.5 Hz, 1H), 2.60 (dt, J =
12.3, 7.5 Hz, 1H), 1.50 (d, J = 6.8
Hz, 3H), 1.37 (d, J = 7.0 Hz, 3H),
1.36 (t, J =
7.5 Hz, 3H), 1.32 (dt, J = 12.3, 8.8
Hz, 1H); C NMR (CDCl): δ 143.4-101.6 (8 signals), 44.8-15.1 (7 signals).
Aryne-based strategy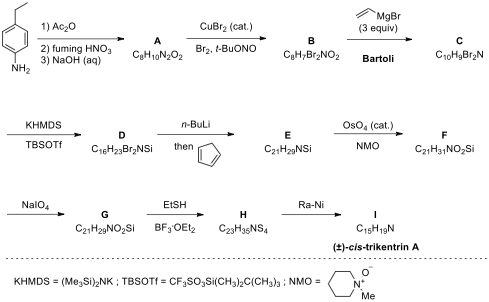 **
**
7.1. Draw the structures of A–I.
7.2. Draw
the structure of the aryne involved as a reaction intermediate in step D → E.
Hydrovinylation strategy
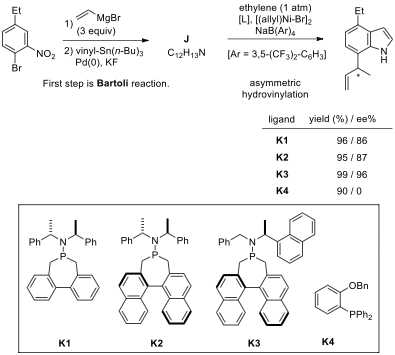
7.3. Chemical transformation of bromo-nitrobenzene
to corresponding 7-vinylindole J includes
in Bartoli reaction followed by the vinylation step with vinylstannane. Draw the structure of J.
7.4. The second step is the Ni(II)-catalyzed
asymmetric hydrovinylation of J. The
ligands (K1–K4) used for
hydrovinylation are given above.
Note: ee = enantiomeric excess; % ee = % major
enantiomer - % minor enantiomer
Choose the correct statement(s):
| ☐ | Ligand 3 gave the best enantioselectivity. | | —- | —- | | ☐ | Ligand 4 gave a racemic mixture. | | ☐ | Each of the ligands K1–K4 is chiral. | | ☐ | Each of the ligands K1–K4 gave excellent yield (>95%) of the product. |
7.5. For the hydrovinylation step, choose the correct statement(s):
| ☐ | (allyl)NiBr or [(allyl)NiBr] is a source of vinyl. | | —- | —- | | ☐ | In this Ni-allyl complex, each nickel has oxidation number +2. | | ☐ | In this Ni-allyl complex, the electron count of Ni is 18. | | ☐ | This complex has a square planar geometry. |
7.6. Draw
the structures of L–P.
The absolute configuration of the asymmetric center in the hydrovinylation
product is S. Hint: In the C NMR spectrum of compound M, one carbonyl carbon signal was
observed at δ = 178.3 ppm.
Key to Problem 7 -by Alec
Correction: The last product need one more Me group, similar to its precursor.
Problem 8. Stereoisomers of 1,2,3-Triphenylpropane-1,3-diol
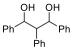
8.1. Draw
all possible stereoisomers of 1,2,3-triphenylpropane-1,3-diol.
8.2. List
all the achiral compounds.
8.3. List
all the chiral compounds.
8.4. Which of the following properties or methods
can be used to distinguish between the chiral compounds from question 8.3? Choose all correct statements.
| ☐ | boiling point |
|---|---|
| ☐ | UV spectroscopy |
| ☐ | refractive index |
| ☐ | melting point |
| ☐ | optical rotation |
| ☐ | dipole moment |
| ☐ | NMR spectroscopy in an achiral environment |
| ☐ | IR spectroscopy |
Problem 9. NMR, Symmetry, and Structural Analysis
Naphthalene
halides: Key compounds for many applications
Besides benzene, naphthalene is one of the best-known aromatic
hydrocarbons. Therefore, the chemistry of naphthalene (1) has been
extensively studied and many naphthalene derivatives have been synthesized.
Halogen derivatives of this kind of compound are key for many transformations.
For this reason, nearly all halogenated derivatives of naphthalene are known in
the literature. Both H and C NMR spectra of symmetric
compounds are characteristic, and allow researchers to exclude possible non-symmetrical
structures to analyze the correct structures. Let us consider naphthalene
tetrabromide isomers 2.

9.1._ _Draw the structures of all naphthalene tetrabromide(s) with 3 signals in C
NMR and one signal (singlet) in H NMR spectra.
9.2._ _Draw the structures of all naphthalene tetrabromide(s) with 5 signals in C
NMR spectra.
9.3._ _Draw the structures of all naphthalene tetrabromide(s) with 6 signals in C
NMR and a doublet (J = 8–9 Hz) in H NMR spectra.
9.4._ _Draw the structures of all naphthalene tetrabromide(s) with 6 signals in C
NMRand a doublet (J = 1.5–2.0 Hz) in H NMR
spectra.
Dynamic NMR: fast
transformation between tautomeric forms and identical nuclei in NMR
Bullvalene (3) is very suitable for degenerate Cope
rearrangements. Without counting enantiomers, the number of possible valence
tautomers of a bullvalene with ten distinguishable positions is 10!/3 =
1,209,600. This arrangement enables all carbon and hydrogen atoms to appear
equivalent on the NMR timescale. At sufficiently high temperature, both H
NMR and C NMR spectra of bullvalene show only one signal, average
to a rounded peak. However, at −60 °C, as Cope rearrangements do not take
place, olefinic and aliphatic protons are observed separately.
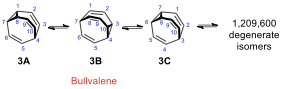
9.5. At low temperature, ignoring any Cope rearrangement, how many
carbon signals do you expect from the C NMR spectrum of
bullvalene?
Label identical carbon atoms with letters a, b, c… on the
molecular structure.
9.6 Owing to fast tautomerism, some molecules give clearer spectra due to apparent
symmetry. In light of this information, how many signals do you expect from the
C NMR spectra of the following compounds?
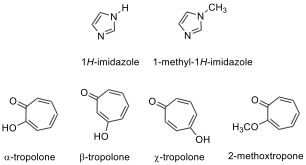
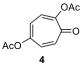
9.7. In the literature, it has been shown that the tropolone diacetate
derivative 4 has fewer signals than expected in C NMR
spectroscopy.
Draw reasonable resonance structure(s) and/or transformation(s) responsible
for this symmetry. How many signals do you expect for this molecule in the C
NMR spectrum?
Stereochemistry of the epoxidation reaction of
bicyclic alkenes.
9.8. Considering the following pieces of information, draw
the structures of all possible stereoisomers formed under the given reaction
conditions.
Hint: A and B are isomers with 3 signals and C is an isomer with 4 signals in C
NMR spectroscopy.
9.9. Draw the structures of the stereoisomer(s) formed under
the given reaction conditions. How many signals do you expect for the epoxide
product(s) in C NMR spectra?
Problem 10. Woodward–Hoffmann Rules and Pericyclic Reactions
The Woodward–Hoffmann rules (or the pericyclic selection rules), developed by Robert B.
Woodward and Roald Hoffmann, are used to rationalize or predict some
stereochemical aspects and the activation energy of pericyclic reactions. They
are for all classes of pericyclic reactions (and their reverse ‘retro’
processes), such as cycloadditions, sigmatropic shift, electrocyclization, ene,
and cheletropic reactions.
| ** | ||
|---|---|---|
| System | Conditions | Motion |
| 4n | thermal |
(∆) | conrotatory (con) | | | photochemical (hν) | disrotatory (dis) | | 4n+2 | thermal | disrotatory | | | photochemical | conrotatory |
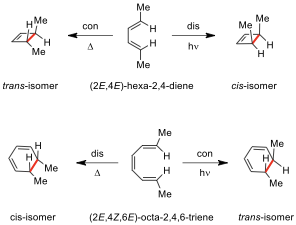
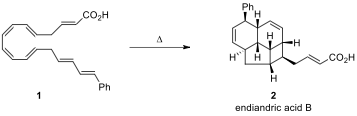
10.1. Thermal reaction of compound 1 results in the formation of
endiandric acid 2 by a series of
pericyclic reactions. Show
all steps and classify their
pericyclic processes.
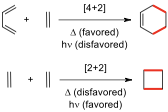
How many π electrons are
involved in the following reactions? Are these reactions thermally or
photochemically allowed according to the Woodward–Hoffmann rules?
10.2.
10.3.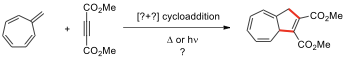
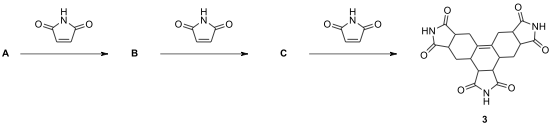
10.4. Domino Diels–Alder reaction of A with succinimide results in the
formation of adduct 3. Draw the structures of A–C.
10.5. The following reaction scheme illustrates the synthesis of endo-isomer
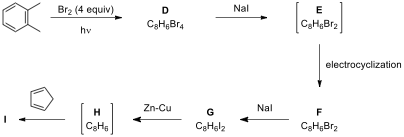
of benzenoid tetracyclic hydrocarbon I starting from o-xylene. Br-elimination
of tetrabromo-o-xylene D with sodium iodide leads to a reactive
intermediate which undergoes a 4 electrocyclization to yield compound F. Draw the
structures of intermediates and products D–I.
retro-Diels–Alder Reaction
The retro-Diels–Alder
(r_DA) reaction is the reverse of the
Diels–Alder reaction, i.e., the formation of diene and dienophile from cyclohexene.
Generally, an _r_DA reaction is
initiated by heating. In some cases, low temperature is sufficient for this
transformation, depending on the nature of the substrate.
10.6. Cyclopentadienes are very useful synthetic intermediates in the fields
of organic and coordination chemistry. Parent (unsubstituted) cyclopentadiene
is obtained by the thermal decomposition of dicyclopentadiene. However, substituted
cyclopentadienes are generally unstable due to the facile migration of the
endocyclic double bonds. Consequently, practical and general methods for the
synthesis of substituted cyclopentadienes are limited. In the following reaction scheme, the synthesis of a substituted
cyclopentadiene derivative is given. Besides _r_DA, some steps also involve the _inverse-Diels–Alder reaction, which is a cycloaddition between an
electron-rich dienophile and an electron-poor diene (such as tetrazine 4), through the interaction of the HOMO
orbital of dienophile and the LUMO orbital of diene.
Draw the structures of the intermediates and
products J–N.

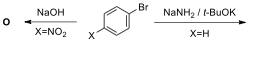
10.7. Nucleophilic aromatic substitution reactions constitute an important
class of reactions in synthetic organic chemistry. In the following scheme, the
reactions of aryl halide 5 proceed via two different kinds of intermediates
in presence of a cyclic 1,3-diene depending on the reaction conditions and the
nature of the substituent on the aromatic ring. Draw the
structures of products (O and P), and discuss
possible intermediates responsible for the formation of these products.
Problem 11. Benzoporphyrin
The name
“porphyrin” derives from the Greek word porphyra, meaning purple. Porphyrins are a group of macrocycle
organic compounds, composed of four modified pyrrole subunits. They have a
total of 26 π-electrons, 18 of which form a planar porphyrin ring structure.
They are often described as aromatic. Metal complexes derived from porphyrins
occur naturally. One of the best-known families of porphyrin complexes is heme,
the pigment in red blood cells. A benzoporphyrin is a porphyrin with a benzene
ring fused to pyrrole unit(s).
11.1.
Benzoporphyrins can be
prepared starting from a masked pyrrole derivative E. The synthesis of E
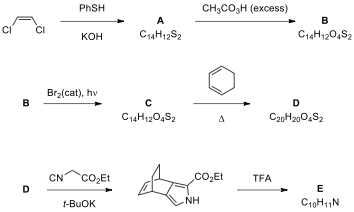
starts with a reaction of cis-1,2-dichloroethene
and thiophenol to give A. Oxidation
of A yields B having phenylsulfonyl units. The cis-product B is then
converted to its trans isomer C when treated with a catalytic amount
of Br under UV light. The Diels–Alder reaction between C and 1,3-cyclohexadiene under thermal
conditions gives the product D,
which is converted to a pyrrole carboxylic acid ester when reacted with ethyl
isocyanoacetate. Ester then is treated with TFA to give the pyrrole derivative E.
Draw the structures of compounds A–E including
stereochemistry when necessary.
11.2. Porphyrins can easily be prepared via a cyclization reaction of pyrrole
derivatives with aldehydes. Draw
the structure of aldehyde F and determine the oxidation state of
zinc in compound H.
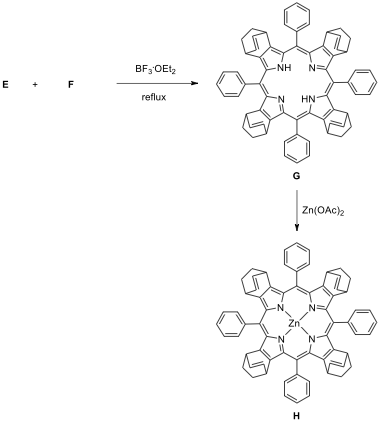
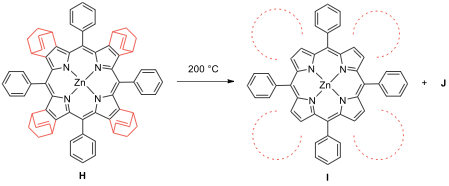
11.3. When H
is heated under vacuum, it can give a more conjugated product through a retro-Diels–Alder reaction.
To complete the structure of I, draw
the structures of the dashed circle part of I (all the circles are identical) and J.
Ammonia is a major metabolic compound and the
importance of its sensitive detection has been emphasized recently because of
its correlation with specific diseases. In normal physiological conditions,
ammonia can be expelled from slightly alkaline blood and emanated through the
skin or exhaled with the breath. Dysfunction in the kidney or liver that
converts ammonia to urea can result in an increase in the ammonia concentration
in breath or urine. Consequently, the detection of the ammonia present in
breath or urine can be used for the early diagnostics of liver or stomach
diseases. The development of sensor devices for measuring ammonia with a
sensitivity of 50 ppb–2 ppm and with a fast response time is highly desired.
For that purpose, I was used to prepare a fiber-optic ammonia gas sensor. Exposure of
this sensor to ammonia changes the transmittance of the fiber-optic. By using
an appropriate spectrometer, ammonia gas in different concentrations was passed
through the sensor and the change in transmittance was measured. The results of
these measurements are listed in the Table below.
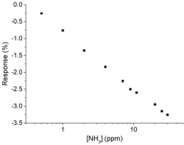 |
[NH] (ppm) | Sensor response (%) | |
|---|---|---|---|
| 0.500 1.00 2.00 4.00 7.00 9.00 11.0 20.0 25.0 30.0 |
–0.2540 –0.7590 –1.354 –1.838 –2.255 –2.500 –2.600 –2.947 –3.152 –3.256 |
| | :—-: | —- |
11.4. Using the linear region of sensor response
data prepare a calibration curve and find
the calibration equation as
.
11.5. This sensor is then used for the detection of
ammonia in human breath. When a kidney patient’s breath was fed into the
sensor, a –3.812% change in the response is observed. Calculate the ammonia concentration in the patient’s breath.
Problem 12. Blue to Green, Turquoise
The beauty of the turquoise
color of Lake Salda, where blue meets white sands, fascinates those who see it.
Lake Salda, in the southern province of Burdur’s Yeşilova district, has been
referred to as “Turkey’s Maldives” in recent years for its white sandy beaches
and turquoise water. In fact, turquoise is an opaque, blue to green mineral
that is a hydrated phosphate of copper and aluminum with the chemical formula
of CuAl(PO)(OH)·4HO,
and is known as a gemstone. The word turquoise dates back to the 17
century and is derived from the French turquois, meaning “Turkish”
because the mineral was first brought to Europe through Turkey, from mines in
the historical Khorasan Province of Persia. Phosphorus, which is also in the
structure of turquoise, is an essential part of
life. Without the phosphates in biological molecules such as ATP, ADP, and DNA,
we would not survive. Phosphorus compounds can be found in the minerals in our
bones and teeth. With few exceptions, minerals containing phosphorus are in the
maximally oxidized state as inorganic phosphate rocks, which are partially made
of apatite, and they are today the chief commercial source of this element.
Phosphate products are used as fertilizers in agriculture. They are also used
in animal feeds, as a leavening agent in baking powder and flour, as an
additive to beverages, and in pharmaceuticals. Industrial uses include water
softening, rust proofing, fire proofing, in insecticides and detergents, and
for the manufacture of elemental phosphorus.

Lake Salda
There are three important allotropes of
phosphorus: X, Y, and Z. However, another form of phosphorus,
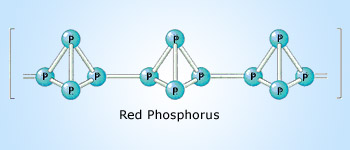
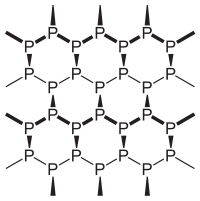
W, also exists (given below). X
is a soft, waxy solid. It is exceptionally harmful and to a great degree
reactive and also displays chemiluminescence. Crystals of X are composed of P molecules. Y is obtained by heating X
to 250 °C within the sight of daylight. It is nonpoisonous and odorless. Y does not show chemiluminescence. It
exists as a polymeric solid. Z is
produced from X under inert
atmosphere. Z is the most stable
allotrope of phosphorus and has a layered structure. W is a form of phosphorus that can be produced by day-long
annealing of Y above 550 °C.
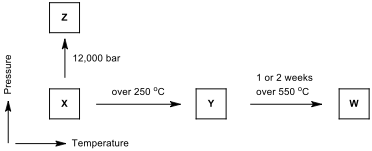
The interconvertible
forms of all allotropes of phosphorus
12.1.
Identify allotropes of
phosphorus indicated by X,
Y, Z, and W.
12.2. Draw the structure of X, Y, Z allotropes of phosphorus and sketch the geometry of X.
12.3. P ignites suddenly in air at
around 35 °C to form a phosphorus oxide derivative. Thus, it is kept under
water. When P reacts with different amounts of dry halogens,
phosphorus trihalides (PX) or phosphorus pentahalides (PX)
are obtained. PX can also be obtained by the reaction of the
halogens with PX. The phosphorus pentahalides undergo hydrolysis in
two steps to form acid. The phosphoryl halides can be prepared by the
hydrolysis of the appropriate pentahalides in a limited amount of water or by
the reaction of the trihalides with oxygen. Dropping of the oxide derivative of
phosphorus into water produces a hissing sound, heat, and acid product. The
reaction of P with sodium or potassium hydroxide produces phosphine
gas as the major product and potassium or sodium hypophosphite as a by-product.
Phosphine burns in chlorine spontaneously, forming a phosphorus trihalide (PX)
or phosphorus pentahalide (PX).
Write the formulas of products A–F.
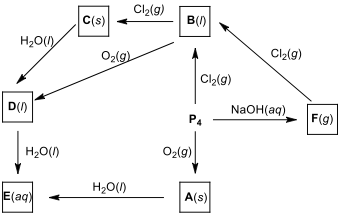
When phosphorus reacts with excess of halogens,
it can form five-coordinated compounds such as PCl. Phosphorus
mixed pentahalides like PFCl are prepared by the
addition of one halogen to the phosphorus trihalide of a second halogen.
12.4. Draw the Lewis structures of PCl and PFCl
molecules.
12.5. By using VSEPR theory, predict the molecular geometries of PCl and PFCl.
12.6. Estimate the polarity of PCl and PFCl
molecules.
12.7. Compare the axial P–Cl bond length to the equatorial
P–Cl bond length in PCl.
12.8. Draw the hybridization scheme of the PFCl
molecule and estimate which
hybrid orbitals are used to form the axial and equatorial bonds.
12.9.
The synthesis of PH
from hydrogen with white phosphorus is given below. Calculate ΔH for the following reaction, using bond energies
(single bond energies (BE) (in kJ.mol) for P–P: 213, H–H: 435,
P–H: 326).
Organophosphorus compounds are organic
compounds containing phosphorus. Phosphorus can adopt a variety of oxidation
states, and organophosphorus compounds are generally classified based on their
derivatives of phosphorus(V) or phosphorus(III), which are the predominant
classes of compounds. Organophosphorus compounds are widely used as
nucleophiles and ligands. Two major applications are as reagents in the Wittig
reaction and as supporting phosphine ligands in homogeneous catalysis. Their
nucleophilicity is evidenced by their reactions with alkyl halides to give
phosphonium salts. Phosphines are nucleophilic catalysts in organic synthesis,
e.g., the Rauhut–Currier reaction and Baylis–Hillman reaction.
Triphenylphosphine (PPh) is a
common organophosphorus compound and it is widely used in the synthesis of
organic and organometallic compounds. When a toluene solution of
compound 1 and excess of PPh
are heated to reflux, first compound 2
is formed and then compound 3.
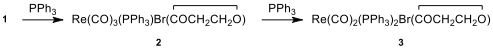
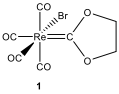
Spectral data of compounds 1–3 are given below (for
H NMR and C NMR data [δ values (relative area)]: 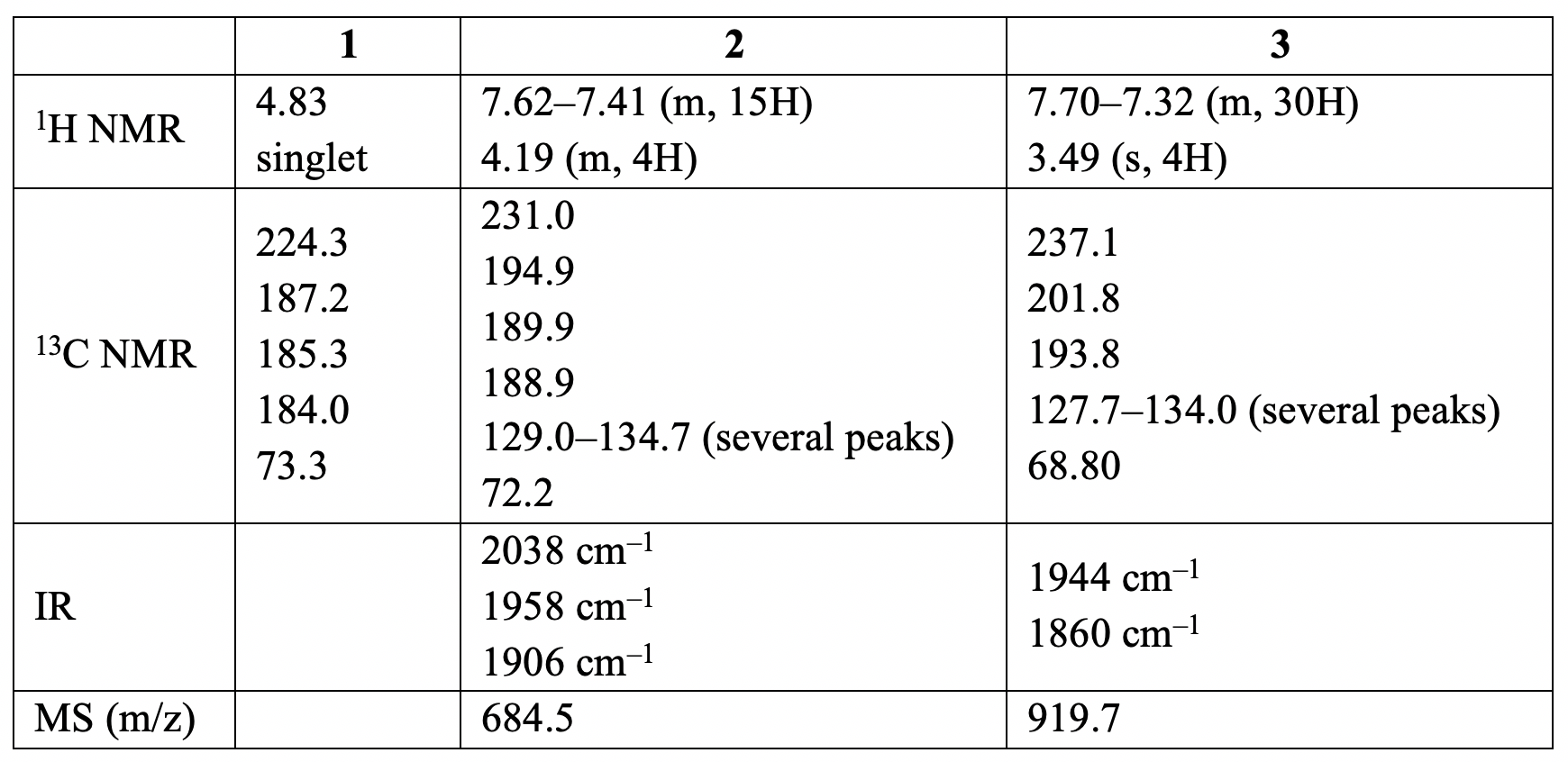
12.10. Identify
the structures of 2 and 3.
Hint: The C NMR
signal of 1 at 224.3 ppm is similar
to the chemical shift observed for carbene carbons; the peaks between 184 and 202
ppm correspond to carbonyls; and the peak at δ 73.3 is typical for CHCH
bridges in dioxycarbene complexes.
12.11. Determine if 2
is more likely to be the facial (fac) or meridional (mer) isomer.
Hint:
The three ν(CO) bands with equal
intensities are observed in the IR spectrum of compound 2. Protons of the carbene ligand occur
as a multiplet in the H NMR spectrum.
12.12. Determine if 3 is
more likely to be cis or trans isomer.
Hint:
The two ν(CO) bands are of approximately equal intensity at 1944 and 1860 cm
in the IR spectrum of compound 3.
The P NMR spectrum shows a single resonance signal.
Some organophosphorus compounds such as sarin, soman, and VX are often referred as “nerve gases”
despite the fact that they are liquids at room temperature. Each country
signing the 1997 Chemical Weapons Convention agreed to ban the development of
chemical weapons and to destroy chemical weapons and associated production
facilities by 2012. Sarin can be destroyed by room temperature hydrolysis using
aqueous NaCO to give NaF and the sodium salt of an
organophosphate. The hydrolysis of nerve agent VX is more difficult. It reacts
slowly with aqueous NaOH at room temperature, and the reaction has to be
carried out at 360 K over several hours.
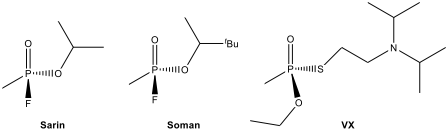
12.13.
Determine the
organophosphorus salt formed in the following hydrolysis reaction.
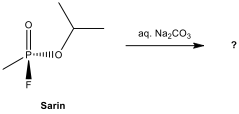
Two chromium complexes containing the ligands
CO, PF, and PCl in octahedral geometry are given below.
In an octahedral complex, the molecular orbitals created by coordination can be
seen as resulting from the donation of two electrons by each of six σ-donor
ligands to the d-orbitals on the metal, called σ-bonding. π-bonding (Pi
bonding) in octahedral complexes is also possible when the ligand has p, d _or π_ molecular orbitals available. Ligands such as CO, CN
and phosphines (of formula PR) are π acceptor, with empty orbitals
that can interact with metal d orbitals in a π fashion. In most cases, the net
back π bonding predominates, and electron density is transferred from the metal
to the ligand. π-bonding can affect metal-ligand bond energy and bond length in
carbonyl and phosphine complexes.
Answer the following questions considering the
π interaction.
12.14. In which complex is the C–O bond shorter, Cr(CO)(PF)
or Cr(CO)(PCl)?
12.15. In the infrared spectrum of which complex do
the C–O stretching bands have *higher
energy, Cr(CO)(PF) or Cr(CO)(PCl)?
Key to Problem 12 by Dr. Chen
12.1 & 12.2
| X | Y | Z | W |
|---|---|---|---|
| white phosphous | red phosphous | black phosphous | purple phosphous |
| soft, waxy, toxic, reactive, chemiluminescent, P4 | nonpoisonous, nonchemiluminescent, polymer | most stable, layer structure, similar to graphite | monoclinic phosphorus, is also known as Hittorf’s metallic phosphorus |
 |
 |
 |
 |
reference: 
https://en.wikipedia.org/wiki/Allotropes_of_phosphorus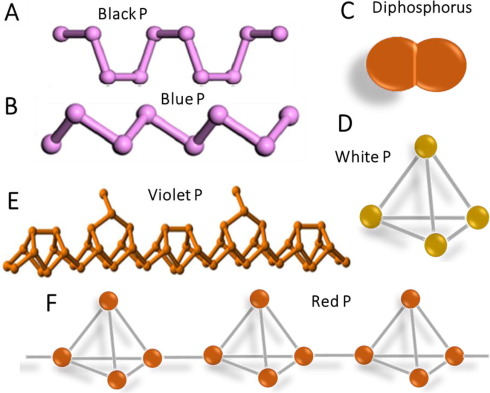
https://www.sciencedirect.com/science/article/pii/S245226271730020X
12.3
Problem 13.
The simple d-block oxides
such as FeO and CoO and many
related mixed metal compounds have important properties. They have structures
related to the mineral spinel, MgAlO, and may be given
a general formula of ABO.
Stoichiometric amounts of two aqua complexes of
transition metal (A and B) nitrate salts are thermally reacted
to form a spinel ABO crystalline solid that has a face-centered
cubic (fcc) structure with a unit
cell composition of 8 ABO. Depending on the location of
these two cations (A and B), the spinel structures are divided into two categories
as normal and inverse spinels. In a normal spinel, the A ions occupy
the tetrahedral holes and the B ions occupy the octahedral holes,
but in the inverse spinel structures, the 2+ ions are replaced by half of the
3+ ions in the structure.
Crystalline
solid has an ordered structure in which the unit cell repeats along all 3
principal axes of a three-dimensional matter. The smallest group of atoms in
the material that constitute this repeating pattern is the unit cell of the structure. The unit
cell completely reflects the geometry and structure of the entire crystal,
which is built up by repetitive translation of the unit cell along the
principal axes. Face centered cubic (fcc)
is one of a common structure type of crystalline solid. Anions (X) are in the
corners and faces of a cube (1/8 from each corners and ½ from each faces,
because the corners and faces are shared by 8 and 2 unit cells, respectively)
in the simplest fcc structure. The
cations (M) occupy the holes among the anions. There are 8 tetrahedral
(corners) and 4 octahedral holes (1 at the middle and 3 on the edges, each edge
has ¼ octahedral hole) in a fcc _structure.
Therefore, the unit cell composition is MX with an empirical
formula of MX. However, the unit cell of a spinel structure is constructed by 8
of these _fcc units.
 **
**
29.746 g salt of A was mixed with 58.202 g salt of B in a thermal process to produce 24.724 g pure product, ABO.
In the spinel formation process, the metal ion of salt A keeps its oxidation
state but the metal ion B undergoes
oxidation. Both salts contain the same number of the water molecule(s), metal
ion, and nitrate ion(s). Elemental analysis of the spinel provided the
following data: 6.538 g metal A and 11.786 g metal B. Assume the end product is
a diamagnetic solid matter. Considering the information provided above. Answer
the following questions.
13.1. Suggest
formulas for the salts of A and B.
13.2. Draw
the structure of one of the complex ions i) without and ii) with one of
the nitrates being in the coordination sphere as a bidentate ligand and identify if the inversion center
is present in the complexes. Inversion is a symmetry operation that translates
every atom through the center to the opposite side.
13.3. Place
the metal ions in an appropriate location in the crystal structure and suggest if it is a normal or
inverse spinel.
The x-ray diffraction data of ABO
provides a unit cell parameter of 8.085 Å, which is constructed from 8 _fcc _units and corresponds to a length of
the edges of the cube.
13.4. Sketch
one of the ffc units of ABO
and place the atoms in the
unit.
13.5 What
is the density of ABO? (hint: 1 Å is 1.0 x 10
m)
Reacting this spinel with other transition
metals (M) produces M doped ABO, where M has a choice
of occupying the place of either A or B-sides. The side product is AO (mono-oxide of A).
13.6. M is Mn
in compound C and Ni in
compound D, suggest the location of Mn and Ni
ions in the structure of C and D, respectively. Assume splitting
energy in Ni and B are 11500 cm and
20800 cm in the octahedral field, respectively, and the pairing
energy is 19500 cm.
If the doping is in a small quantity or in some
cases the doped metal ion behaves like a free ion in the lattice (it means,
electrons of M only feel the surrounding atoms and localized to M and its 1
shell of atoms in the structure). Assume Mn is behaving like a
free ion in the lattice and creating its own localized electronic energy
levels.
13.7. Draw**
the d-orbital splitting and identify if the Mn species are
paramagnetic or diamagnetic.
Magnetic susceptibility could be calculated
from the spin only formula:
where n is a number of unpaired electrons.
However, some other electronic couplings affect the magnetic moment such that a
correction term is needed. The correction term α is related to ground state
(where α = 4 for a non-degenerate and 2 for a degenerate ground state,
degeneracy of a ground state can be determined from the electron
configurations, such as completely filled and half-filled set of orbitals
creates a non-degenerate and a partially filled set of orbitals create
degenerate states) and λ = 88 cm for Mn and -315 cm
for Ni), and splitting energy (Δ is 5000 cm for Mn
and 11500 cm for Ni) and the magnetic moment is:
,
The magnetic susceptibility can be experimentally
determined and it is interrelated with the magnetic moment (if we ignore the
diamagnetic contributions) with the
following formula:
where T is temperature in Kelvin and X
is the molar magnetic susceptibility.
13.8. What is the magnetic
susceptibility of the products at 25 C, if the samples C and D
weigh 25.433 and 25.471 g, respectively (each obtained from 24.724 g ABO)?
13.9. Place all
the metal ions (A, B, Mn, and Ni) into their
appropriate locations in the lattice and fill
up the following table. Use
t for d, d, and d and e
for d, d orbitals in octahedral (O)
and t and e orbitals in tetrahedral (T) cases. If there
is distortion, predict the
type of distortion(s) and show
the d-orbital splitting.
| M | Local geometry | Electron configuration | Degeneracy | Type of distortion |
|---|---|---|---|---|
Problem 14.
Medicinal inorganic chemistry based on metal‐based drugs is broadly defined as the area of research related to metal ions and metal complexes and their clinical applications. It is a new research area that developed from the discovery of the anticancer agent cisplatin. Cisplatin, cis-diamminedichloroplatinum(II), is a yellow powder and an anticancer drug widely used in the treatment of a variety of tumors, especially those of the testes, ovaries, head, and neck.
The synthesis
of cisplatin starts with K[PtCl], but has undergone
several improvements since it was published more than 100 years ago. The main
problem is the occurrence of impurities and the formation of the by-product trans-platin. Nowadays, the synthetic
routes are mostly based on a method published in the 1970s by Dhara. In the
initial step, K[PtCl] is reacted with excess KI, and
the platinum complex is converted into the iodo analogue (A). Subsequently, NH is added to the compound A and compound B is formed by ligand exchange in which two NH ligands
are exchanged with two iodo ligands. B
is a yellow solid that is filtered, dried, and mixed with the aqueous solution
of AgNO. The insoluble AgI can be filtered off and cis-diamminediaquaplatinum(II)
nitrate (C) is formed; then excess
KCl is added to the solution of C to
yield cisplatin (D).
The success of
the synthesis relies on the strong trans
effect of the iodo ligands. The spectator ligands T that are trans _to the leaving group in
square-planar complexes influence the rate of substitution. This phenomenon is
called the _trans
_effect. Key point is
that a strong σ-donor
ligand or π-acceptor ligand greatly accelerates substitution of a ligand situating
in the trans position. _Trans _effects follow the order given below.
For
a T σ -donor: OH- < NH < Cl- < Br- < CN-, CH-< I- < SCN- < PR,
H-
For
a T π-acceptor: Br- < I- < NCS- < NO- < CN- < CO, CH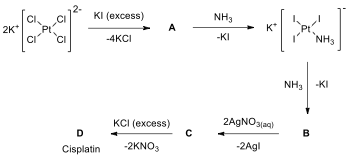
14.1. Write the formulas of
A–D.
14.2. Draw
molecular structures of A–D.
14.3. Is the complex D
polar?
14.4. Sketch the d-orbital splitting of cisplatin complex D in view of Crystal Field Theory and
show the electron distribution diagram.
14.5. Determine
magnetic nature of complex A.
The platinum complex binds to DNA and causes cross-linking, which
triggers the programmed cell death (apoptosis). However, the other geometrical
isomer of the square planar structure transplatin, _trans-diamminedichloroplatinum(II) (F),
is not effective for the treatment of cancer. Transplatin is synthesized
starting from [Pt(NH)] to which the first
and second Cl ligands are added to form transplatin (F) as represented in the scheme below.
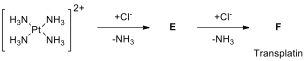
14.6. Draw the molecular structures
of E and F.
The most
important classes of antitumor agents, cisplatin, carboplatin, and oxaliplatin
as platinum(II) diamines are widely used in chemotherapy to treat a wide
variety of cancers.
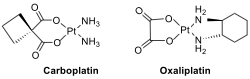
However, the
therapeutic index of these agents is relatively narrow; their use is often
plagued with severe toxicity and the development of resistance, which leads to
disease progression. Recently, oxoplatin, iproplatin, ormaplatin and
satraplatin are Pt complexes that have been used clinically (oxoplatin) or in
clinical trials.
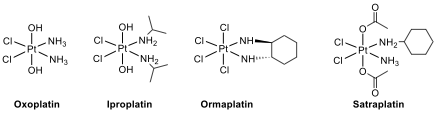
14.7. All complexes have the same geometry and
oxidation number for the Pt central atom.
Write
the
oxidation state of Pt and geometry
of the complexes.
14.8. Which Pt complex, cisplatin or satraplatin, is
kinetically more inert for substitution reactions?
14.9 Oxaplatin is an isomer of [Pt(NH)Cl(OH)]
complex. Draw all
stereoisomers and indicate
the chiral one(s).
Platinum complexes (oxoplatin, iproplatin,
ormaplatin, and satraplatin) can be considered prodrugs that are primarily
intracellularly activated by biological reducing agents such as thiols,
ascorbic acid, and glutathione (GSH) to kill cancer cells.
In a study, for example, the reduction of cis,trans,cis-[PtCl(OCOCH)(NH)]
(G, prodrug), which has a similar
structure to satraplatin, by aqueous extract of cancer cells (A2780, A2780cisR,
and HT-29) yields cisplatin (D,
drug) and free acetate ion as given below.
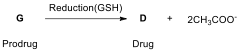
14.10. Draw
the molecular structure of G.
14.11. Sketch the d-orbital splitting of the metal ion in G and write the electronic configuration.
14.12. Decide
whether G is paramagnetic or
diamagnetic.
14.13. The complex G crystallizes
into a monoclinic crystal system of parameters: the lengths of the unit cell: a = 14.9973, b = 8.57220, c = 11.1352
Å, the β angle in the unit cell = 126.7690°, the number of the
molecules in the unit cell (Z) = 4, M = 436.16 g/mol (the complex has
one water molecule in the crystal structure).
Calculate the density (ρ) of the complex.
Hint: the volume of a monoclinic crystal unit cell is
Problem 15.
The Salt Lake
basin in Turkey is of great importance for the conservation of biological
diversity and is classed as a wetland according to international criteria. It
is also one of Turkey’s richest lakes for the presence of birds. There are 85
bird species, 129 insect species (4 of which are endemic), 15 mammal species,
and 38 endemic plant species. Some 40% of Turkey’s salt needs (as table salt)
are supplied from this lake. Salt in the Salt Lake is formed by meteorological
waters draining underground and melting the previously formed salt domes and
carrying them along the tectonic lines. Salt production in the Salt Lake is
done by evaporation of lake water under the sun. A pooling system is used in
the salt production with solar energy.

Salt Lake
Table
salt is one of the most common household chemicals. It is 97% to
99% sodium chloride, which is an ionic
compound with the chemical formula NaCl, representing a 1:1 ratio of sodium and chloride ions. NaCl is the compound most responsible
for the salinity of seawater and of the extracellular fluid of many multicellular
organisms. In its edible form of table salt, it is commonly used as a condiment
and food preservative. A second major application of sodium chloride is
de-icing of roadways in subfreezing weather. Large quantities of sodium
chloride are also used in many industrial processes such as the chloro-alkaline
industry and soda-ash industry as well as in miscellaneous industrial uses: water
softening, medicine, agriculture, firefighting, and cleanser. NaCl is used, directly or indirectly,
in the production of many sodium compounds, which consume most of the world’s
production. The scheme below shows the preparation of some sodium compounds
starting from NaCl.
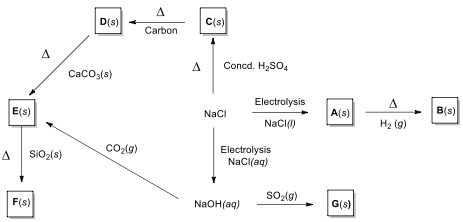
Preparation of some sodium compounds starting
from NaCl.
15.1. Write
the formulas of products A–G.
Sodium carbonate (NaCOsoda ash) is used
primarily in the manufacture of glass, which is produced mostly from natural
sources, such as the mineral trona, NaCONaHCOnHO. It can be
also manufactured mostly from NaCl, CaCO, and NH using
a process introduced by the Belgian chemist Ernest Solvay in 1863. The key step
involves the reaction of NH(g) and CO(g) in saturated
NaCl(aq). Of the possible ionic compounds that could precipitate from such a
mixture (NaCl, NHCl, NaHCO, and NHHCO),
the least soluble is sodium hydrogen carbonate (sodium bicarbonate,
NaHCO). It is isolated from solution by filtration and then
converted to sodium carbonate (NaCO) by heating.
According to this explanation,
15.2. Balance the reactions given below.
15.3. Using CaCO
(limestone), how can you produce the CO gas you need to prepare NaHCO?
15.4. Write the Lewis structure of CaCO with all
resonances and show formal
charges for each atom in the structure.
15.5. Describe the molecular geometry and propose
a plausible hybridization scheme for the central atom in the ion CO.
15.6. Compare the bond lengths of CO, CO,
and CO in increasing order.
NaCl
crystallizes in a face-centered cubic (fcc) structure. The density of NaCl is
2180 kg/m and the ionic radius of Na is 99 pm.
15.7. How many atoms are there in the unit cell? Which atoms occupy octahedral
holes?
15.8. Calculate the length of the unit cell of NaCl and the ionic
radius of the Cl ion (as pm).
15.9. The alkali metals react rapidly
with oxygen to produce several different ionic oxides. Under appropriate
conditions, generally by carefully controlling the supply of oxygen, the oxide
MO can be prepared for each of the alkali metals. Lithium reacts with
excess oxygen to give ……(A)…. and a
small amount of ……(B)……. Sodium
reacts with excess oxygen to give mostly ….(C)……
and a small amount of ……(D)……..Potassium,
rubidium, and cesium react with excess oxygen to form …….(E)….., ……(F)….., and ……(G)…..
Fill in the blanks above (for A–G) with convenient
formulas of metal oxides.
15.10. Draw the Lewis structures of oxide,
peroxide, and superoxide ions.
15.11. Draw the molecule orbital energy level diagram of peroxide
and superoxide ions and compare their
bond lengths and energies.
When LiClO,
NaClO, and KClO crystallize from an aqueous solution
that may or may not contain water molecules called water of crystallization as
part of the solid structures, although no simple rule exists for predicting with
certainty whether the ions will retain all or part of their hydration spheres
in the solid state, cations with high charge densities tend to retain all or
part of their hydration spheres in the solid state. When the cations have low
charge densities, the cations tend to lose their hydration spheres; thus, they
tend to form anhydrous salts. The ionic radius of Li, Na,
and K is 76 pm, 102 pm, and 138 pm, respectively.
~~ ~~
15.12. Calculate the charge densities of the ions in C
mm.
15.13. Which perchlorate salt
is most susceptible to form an anhydrous compound?
Problem 16.
Turkey is one of the 7 countries in the world in terms of thermal source
richness with almost 1300 thermal springs throughout Anatolia. There are thermal hotels in many cities such as Ankara, Bursa,
Balıkesir, Yalova, Erzurum, Sivas and Afyonkarahisar. Afyonkarahisar, located
in the Aegean region, is the most famous city in Turkey for its thermal
springs. The thermal waters of Afyonkarahisar contain over 42 different
minerals and many trace elements. The most concentrated ones are sulfur,
calcium, chloride, sodium, and carbonates. Among these minerals, sulfur is
important as “nature’s beauty mineral” because the human body needs it to
manufacture collagen, which keeps human skin elastic, beautiful, and young
looking. Moreover, sulfur is used to minimize the symptoms of many skin
diseases including dermatitis, eczema, dandruff, and warts. People with
arthritis may obtain pain relief from taking a soothing bath in thermal sulfur
springs. Mineral water containing sulfur compounds is also shown to decrease
cholesterol and blood pressure. Therefore, sulfur chemistry is an important
topic. In this question, you will explore sulfur chemistry by studying its
different reactions and compounds.

Hot spring
Sulfur is extracted as the element from underground deposits. It has
many allotropes and its allotropy is complicated, but the most common sulfur
allotrope is the puckered rings of S (orthorhombic sulfur, -form).
16.1. Sketch the molecular structure of S
and indicate whether the molecule has a horizontal mirror
plane or not.
Upon the burning of S with oxygen, compound A is
produced. Further catalytic oxidation of compound A yields compound B.
The reaction of A and B with water (hydrolysis) yields C and
D. Compound D is an oxoacid and a central substance of the chemical
industry worldwide.
16.2. Write the formulas of compounds A–D.
16.3. Draw molecular shape of the compounds by giving the name of geometries.
16.4. Write the oxidation state of the sulfur atoms in C and D.
16.5. Give balanced chemical equations for the synthesis of A–D.
Compound A can also be obtained by heating alkaline or alkaline
earth sulfide minerals like CaS in an excess of air.
16.6. Write the balanced
chemical equation for the synthesis of A from CaS.
Upon the reaction of D and B, compound E which is a
dense and corrosive liquid that is used as a basic chemical for sulfonation
processes is produced.
16.7. Give a balanced chemical equation for the synthesis of E from D.
16.8. Write molecular formula and draw the molecular shape of E.
16.9. Determine the oxidation state of the sulfur atoms in E.
The reaction
of S with a stoichiometric amount of chlorine gas yields compound F
and the further reaction of F with excess chlorine gas results in the
formation of G, which is used as a precursor for the
synthesis of sulfur dyes and synthetic rubber. The reaction of G with B
yields the compounds H and A. H is a toxic compound
used as the chlorinating agent in organic synthesis.
16.10. Write molecular formulas and draw the molecular shapes of F, G, and H.
16.11. Give balanced chemical equations for the synthesis of compounds F, G, and H.
One of the
most common naturally occurring sulfur minerals is pyrite (FeS:
iron(II) disulfide), called fool’s gold because it is a brass-yellow mineral
and thus most people suppose that it is gold ore. The treatment of pyrite with
hydrochloric acid results in the formation of a colorless, flammable, water-soluble
gas with a “rotten egg” odor, compound I. Compound I is dissolved
in thermal waters for spa applications since it is reported that the
therapeutic effects of thermal water are directly correlated to its sulfur
concentration. Compound I is slightly heavier than air and can be
detected by lead(II) acetate paper strip test in which a reaction occurs
between lead(II) acetate and I, producing compound J. Moreover,
upon the oxidation of I, compound A can be yielded.
16.12. Write the molecular formulas of I and J.
16.13. Draw the molecular shape of I and write the name of the shape.
16.14. Give balanced chemical equations for the synthesis of I and J.
The sulfur oxoacids are chemical compounds that contain sulfur,
oxygen, and hydrogen atoms. Sulfur has several oxoacids; one of them is
thiosulfuric acid, with the molecular formula HSO,
which can be synthesized by the reaction of sulfite with I. On the other
hand, the controlled oxidation of sulfite by MnO in acidic solution
yields another sulfur oxoacid, called dithionic acid, HSO.
16.15. Give balanced chemical equations for the synthesis of HSO
and HSO.
16.16. Draw the molecular shapes of HSO
and HSO.
On the other hand, the thiosulfate ion (SO)
is a very good complexing agent for Ag and thus it is used in
photography for removing unchanged AgBr from exposed photographic film. Upon
the reaction of sodium thiosulfate ion with AgBr, sodium salt of a coordination
compound with coordination number 2 is yielded.
16.17. Give a balanced chemical equation for
the reaction of AgBr with NaSO.
16.18. Draw the molecular structure of the yielded coordination complex considering
its geometry.
16.19. Write the electron configuration of the silver ion in the coordination
compound.
The determination of HS content in
thermal waters is important for spa applications. An iodometric titration
method can be utilized for this purpose. In a typical experiment, 500 mL of
sample is collected from a thermal water source and purged with N(g)
to ensure the transfer of all HS gas into 50 mL of 0.010 M NaOH
solution in a closed system. After adjusting pH of the solution to 6.0, 1.25 mL
of 0.0030 M I solution and 1.0 g of KI are added to this solution
and the resultant solution is stored in the dark for 15 minutes after sealing
it with parafilm. After adding 1.0 mL of 20 mg/mL starch solution, the
resultant solution is titrated with 0.0500 M NaSO
until the end-point and consumed NaSO
volume is recorded as 95.62 mL.
16.20. Write all balanced equations of this experiment.
16.21. Calculate HS concentration in the thermal
water source in ppm by assuming that there is no interfering species in the
water source and all HS content of thermal water is swept into the NaOH
solution.
Problem 17.
Flavonoids are a group of
natural products with some phenolic groups that are present in many fruits and
vegetables. Flavonoids are commonly used in our daily life due to their
antioxidant and anticarcinogenic properties. Rutin is a flavonoid class
substance composed of the flavonol quercetin and disaccharide rutinose.
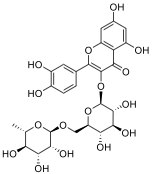
Chemical structure of
rutin.
It is of very low toxicity for human health and
it is known that rutin can supply electrons to reactive free radicals to
produce more stable and healthy structures. Rutin is also known as vitamin P,
in which P is due to its permeability.
Rutin is an electrochemically active material and many researchers have
extensively studied its electrochemical behavior using different
electrochemical techniques.
Cyclic voltammetry is a useful technique for
electrochemical measurement of an analyte, which is dissolved in a useful
electrolyte solution. There are three electrodes in an electrochemical cell
solution; working, counter, and reference electrodes. The potential of working
electrode is scanned versus reference electrode because reference electrode has
a constant potential value. Reverse electrochemical reactions of the working
electrode occur at the counter electrode. Therefore, current flows between
working and counter electrodes. Reference electrode is used to adjust the
potential of the working electrode at a known value. This technique is applied
based on potentiodynamic application. Potential of the working electrode is
scanned versus reference electrode between two potential values depending on
time. Cyclic voltammetry application results in a graphic (voltammogram) of
current versus scanned potential. There are two important parameters to
evaluate a voltammogram; peak potential and peak current. The peak potential
and peak current are calculated using x-axis and y-axis of a voltammogram at
the peak maximum, respectively.
The cyclic voltammetry (CV) behavior of rutin
at 25 °C has been tested using a glassy carbon electrode, a saturated calomel
electrode (SCE), and a Pt wire as working, reference, and counter electrodes,
respectively. In this study, CV data for 1.0 × 10 mol/dm
rutin solutions at different pH values have been obtained by scanning the
potential between 0.00 and 0.80 V at a scan rate of 100 mV/s. Anodic peak
potential (Ep), cathodic peak potential (Ep), anodic
peak current (Ip), and cathodic peak current (Ip)
values supplied from related CVs depending on the pH are presented in the
following Table.
Table. Some CV parameters depending on the pH of a solution containing 1.0×10
mol/dm rutin.
| pH | Ep/mV | Ep/mV | Ip/A** | Ip/μA |
|---|---|---|---|---|
| 1.5 | 643 | 614 | 0.105 | –0.104 |
| 2.0 | 609 | 578 | 0.118 | –0.119 |
| 3.0 | 544 | 514 | 0.116 | –0.117 |
| 4.0 | 499 | 470 | 0.104 | –0.104 |
| 5.0 | 441 | 410 | 0.093 | –0.092 |
| 6.0 | 372 | 344 | 0.099 | –0.100 |
17.1. In a three-electrode system, electrochemical
oxidation or reduction of an analyte in the electrochemical cell occurs on the
__ because its potential is adjusted against the
_.
Which of the
following words fit into the blanks in the above sentence?
a)
working
electrode / reference electrode
b)
counter
electrode / working electrode
c)
reference
electrode / working electrode
d)
working
electrode / counter electrode
17.2. Both anodic and cathodic peak potentials shift to negative potential
values by increasing the pH because the electrochemical reaction of rutin
includes __.
Which of the
following words fits into the blank in the above sentence?
a)
Na
b)
K
c)
H
d)
I
17.3. Electrochemical oxidation of rutin is _ because of the
fact that Ip/Ip is about 1 and ΔE_p is almost 0.0592/_n
V.
Which of the
following words fits into the blank in the above sentence?
a)
irreversible
b)
reversible
c)
quasi-reversible
d)
catalyzed
17.4. How long does it take to obtain each CV value?
17.5. Calculate the number
of transferred electrons for the electrochemical reaction of rutin including 2
H.
17.6. Propose an
electrochemical redox mechanism for rutin.
17.7. The SCE reaction is and the SCE
contains saturated KCl solution prepared by dissolving 342 g of KCl in 1.0 L of
aqueous solution. How does the potential of the SCE change (decrease or increase) in the case of 1.0
M KCl?
In order to
determine the amount of rutin in a vitamin P tablet, the following procedures
have been used:
i) A 500 mg vitamin P tablet is dissolved in deionized water, pH is
adjusted to 2.0, and total volume is completed to 500 mL in a volumetric flask.
A 10 mL part of this solution is placed in a three-electrode cell. CV is
obtained with an anodic peak current (Ip) of 2.26 A.
ii) A solution in the absence of rutin has been prepared at pH 2.0. After
placing all electrodes into this solution, CV has been recorded three times by
cleaning the electrode with deionized water for each measurement. Then Ip
values have been read as 0.16, 0.11, and 0.18 μA,
respectively.
iii) The standard rutin solutions of 1.0, 5.0, 10.0, 20.0, 30.0, and 50.0 mM
have been prepared and Ipvalues of these solutions have been
obtained from related CVs as demonstrated in the following Table.
Table. Ip values for various rutin
standard solutions.
| C/mM | IpA |
|---|---|
| 1.0 | 1.11 |
| 5.0 | 6.43 |
| 10.0 | 12.62 |
| 20.0 | 24.73 |
| 30.0 | 36.20 |
| 50.0 | 58.55 |
Note that all
of the CVs have been obtained by using same working electrode beyond this
experiment.
17.8. Draw a calibration curve for the rutin
determination method.
17.9. Write a mathematical equation for the calibration
curve.
17.10. Calculate the rutin
amount in the vitamin P tablet as wt %.
17.11. Calculate the
calibration sensitivity and limit of detection (LOD) of this method for a
signal to noise ratio (S/N) of 3.0.
Note: Limit of detection:
Problem 18.
The particle in an one-dimensional box model is a crude approximation for conjugated molecules. In this model, π electrons are assumed to move freely over the carbon framework of conjugated bonds. Therefore, this model is also called the free electron model (FEM). The length of the box may be approximated via
, where L is the box length and
is the number of carbons. Furthermore, the
Pauli principle is applied when electrons are filled to the energy levels. The
energy of a particle in an one-dimensional box can be written as follows:
where m is the mass of the particle, h is the Planck constant, and n is a positive integer.
For the 1,3,5,7-octatetraene molecule assuming FME:
18.1. Draw an energy diagram, fill the electrons, and calculate orbital energies.
18.2. Calculate the total π energy of the molecule.
18.3. Determine the wave
length of the light (in nm) that require to excite an electron from the highest
occupied molecular orbital (HOMO) to the
lowest unoccupied molecular orbital (LUMO).
For two-dimensional conjugated systems, we may use the
particle in a two-dimensional box model. In this case the total energy can be
written as follows:
where _L_1 and _L_2
are the lengths and
and
are the quantum numbers of the first and
second dimensions, respectively.
Graphene is a sheet of carbon atoms in the form of a two-dimensional
hexagonal lattice in which one atom forms each vertex.
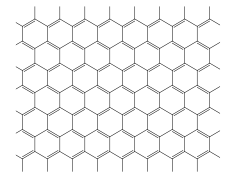
For a square shaped graphene sheet with L_1 = _L_2 = 11 Å:
18.4. The
distance between two adjacent carbons in the hexagonal 6-carbon unit is
approximately 1.4 Å. Calculate
the number of electrons in a (×)
sheet of graphene. For this problem you may ignore edge electrons. (Area of a
regular hexagon with a side of _L is
).
18.5. Calculate the energy of the HOMO.
18.6. Calculate the energy of the LUMO.
18.7. The difference between energies of
the LUMO and HOMO is called the band gap (E). Calculate
the band gap.
The models for a particle in a one- and two-dimensional box can be
extended to a three-dimensional rectangular box of dimensions
,
, and
, yielding the following expression
for the allowed energy levels:
where _n_1, _n_2, and _n_3 are the quantum numbers of the first, second,
and third dimensions, respectively. For a particle in a cubic box of length
:
18.8. Give the expressions for the five different lowest energies.
18.9. Draw a diagram showing all the five energy levels. Indicate
degeneracy of each level.
Problem 19.
Vibration of a diatomic molecule is
reminiscent of two masses on a spring with a potential energy that is a
function of the displacement from equilibrium. Hence, the harmonic oscillator
model is utilized to compute vibrational frequencies. These frequencies are
called harmonic vibrational frequencies. The energy of a harmonic oscillator
can be written as follows:
where ν is the harmonic vibrational frequency, h is the Planck constant, and n is a nonnegative integer. The harmonic
vibrational frequency can be calculated as follows:
where k is the force constant and μ is the reduced mass:
where m_1 and _m_2 are the masses of the first and the second
atoms, respectively.
For the CO molecule the value of the force
constant is 1902.4 N/m. For this problem, the atomic masses of isotopes can be
approximated by their mass numbers.
19.1. Calculate the harmonic vibrational frequency of the CO
molecule in Hz.
19.2. Express the harmonic vibrational frequency of the CO
molecule in cm.
19.3. Calculate the zero-point vibrational energy (ZPVE) of the CO
molecule in kcal/mol.
19.4. Calculate the
harmonic vibrational frequency of the CO molecule in
cm.
19.5. Calculate the
harmonic vibrational frequency of the CO molecule in
cm.
The harmonic oscillator model can
readily be extended to polyatomic molecules. In this case, the total
vibrational energy of a molecule with _n vibrational
frequencies can be written as follows:
where νi are the harmonic vibrational frequencies, h is the Planck constant, and ni are nonnegative integers.
For the water molecule the harmonic vibrational frequencies are 1649,
3832, and 3943 cm. Using the harmonic oscillator model, for the
water molecule:
19.6. Calculate the ZPVE value (in J and cm
units).
19.7. Calculate the first 5 energy levels (in cm).
To describe the rotational motion of a diatomic molecule, the rigid
rotor model is used. In this model the bond length (R) of the diatomic molecule is kept
constant during the rotational motion. Using the rigid rotor model, the
rotational energy of a diatomic molecule can be written as follows:
where I is the moment of inertia and l is a nonnegative integer. The moment of
inertia can be written as follows:
where μ is the reduced mass and R is the bond length of the diatomic molecule.
In the microwave spectrum of the CO molecule
the value of frequency for the lowest energy transition is 115.270 GHz.
19.8. Calculate the bond length of the CO molecule in Å.
19.9. For the CO molecule predict the
frequency of the next two absorptions (selection rule is
.
19.10. For the CO
molecule, calculate the frequency of the lowest energy
absorption.
Problem 20.
In the future, humankind
will most likely consume all resources that are necessary for life on earth and
will have to relocate to an earth-like planet. Assume that you have started to
live on a new planet where standard pressure condition is 2 bar, standard
concentration is 1 mol dm, and all types of gases behave as an
ideal gas. On this planet, you are asked to determine equilibrium conditions
for the reaction below:
20.1. Calculate the change in standard enthalpy of the reaction at 298 K by using the
following information:
| X4Y8(s) = 4X(g) + 4Y2(g) |
| | —- | —- | |
|
| |
|
| |
|
|
20.2. Calculate ΔG° of the reaction at 298 K.
20.3. Calculate K° of the reaction at 298 K.
20.4. Assume that ΔH°
of the reaction does not depend on temperature. Find K of the reaction at 50 °C.
20.5. Calculate the percent degree of dissociation for XY at 298 K where
total pressure is 0.2 bar.
20.6. In order to
increase the amounts of products, which one do you choose to increase (if you choose both,
put a cross next to both of them):
| ☐ | pressure |
|---|---|
| ☐ | temperature of the reaction vessel |
Moreover, in this future, the Earth will have a
very unstable climate. The surface temperature could increase or decrease all
of a sudden. Suppose that you travelled through time to the era in which the Earth’s
climate is extremely unstable. Your task in this era is to observe the thermodynamics
of phase transitions of water, the most precious substance where all life has
originated. Suppose that the temperature suddenly decreased to −20 °C.
One mole of water becomes supercooled liquid
water at −20 °C and 1 bar pressure and then turns into ice at the same
temperature (note that the temperature of the surroundings is constant at −20
°C).
By using the following data for water:
The heat of fusion (ΔH°) of ice at 0 °C and 1 bar is 6020 J
mol
During the conversion of supercooled liquid water to ice at −20 °C:
20.7. Calculate the total entropy change in the system.
20.8. Calculate the total entropy change in the surroundings.
20.9. Calculate the total entropy change in the universe.
and