IChO2008PP-Budapest-Q11L3
On January 30 in 2000, a dam failure in a gold mine spilled about 100 000 m3 of cyanide-containing waste water into the river Szamos. The pollution wave, which later reached the Central European rivers Tisza and Danube, killed massive amounts of fish. On February 15, a popular Hungarian TV news show presented a simple experiment: first a NaCN solution was prepared, the concentration of which was similar to those measured in the pollution wave. Fish were killed in this solution but survived when ferrous sulfate was also added. The TV show suggested that ferrous sulfate should have been used to lower the environmental impact of the cyanide solution. However, when the same experiment was repeated with an actual sample from the pollution wave, fish were killed even after ferrous sulfate was added. Unfortunately, this second experiment was not covered in any evening news.
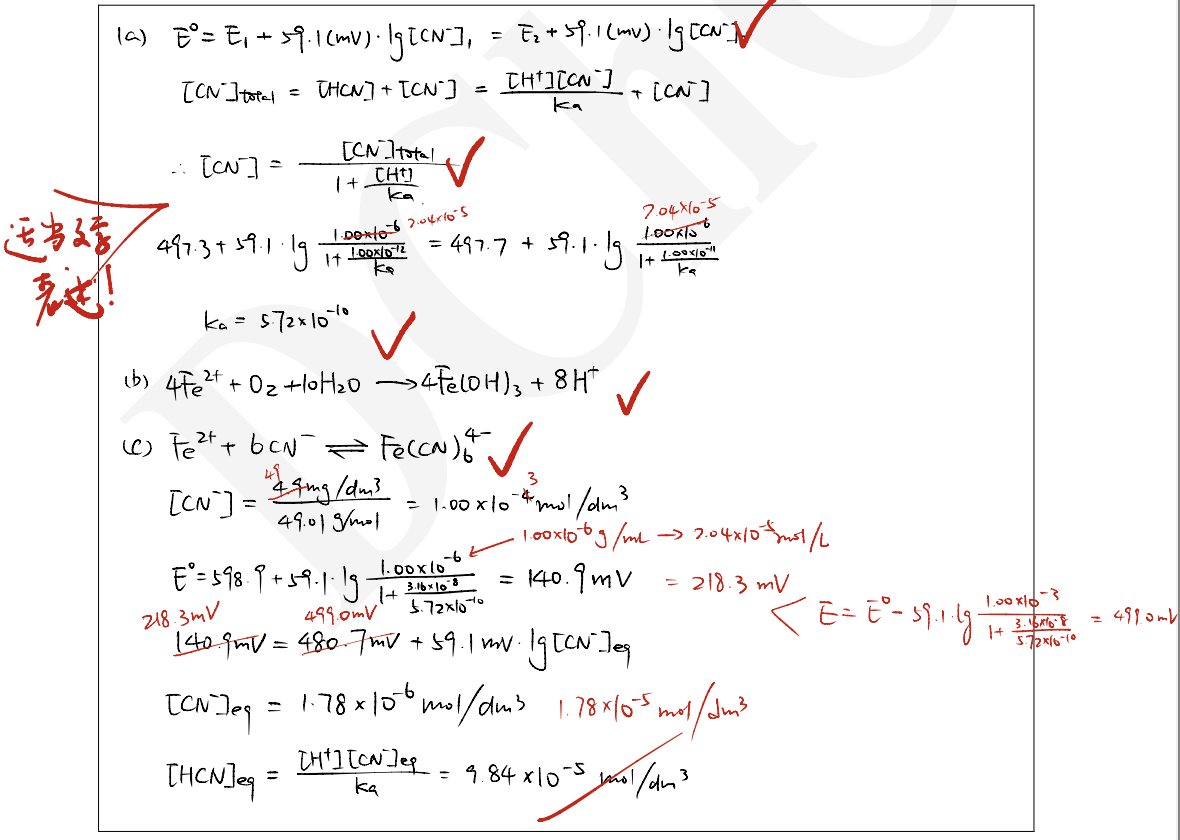
To clarify the underlying chemistry, an expert designed a detailed series of experiments in which the use of a cyanide selective combination electrode was an important element. He first calibrated the electrode using 3 different concentrations at 3 different pH values. The temperature was 25 °C in all experiments. The instrumental readings were as follows:
ppm= parts per million (g/g)
| 1.00 ppm NaCN | 10.0 ppm NaCN | 100 ppm NaCN | |
|---|---|---|---|
| 0.01 mol/dm3 NaOH | 497.3 mV | 438.2 mV | 379.1 mV |
| 0.001 mol/dm3 NaOH | 497.7 mV | 438.6 mV | 379.5 mV |
| pH = 7.5 buffer | 598.9 mV | 539.8 mV | 480.7 mV |
a) Calculate the acid dissociation constant of HCN based on these measurements.
To 100 cm3 of a test solution, which contained 49.0 mg/dm3 NaCN and was buffered to pH = 7.5, 40.0 mg of solid FeSO4·7H2O has been added. At this pH, the reaction between aqueous iron(II) and dissolved oxygen is quantitative under all conditions and gives an iron(III) hydroxide precipitate. Ignore possible complexation reactions between the precipitate and cyanide ions.
b) Write the balanced equation for this redox reaction.
All the solutions used in the experiments initially contained 8.00 mg/dm3 dissolved oxygen. The electrode reading in this solution was 585.9 mV. Iron(II) only forms one complex with cyanide ion, which has a coordination number of 6.
c) Write the ionic equation describing the formation of this complex. Estimate the stability constant of the complex.
The following toxicity data (LC50: median lethal concentration for 24-hour exposure) for fish can be found in tables:
| LC50 | |
|---|---|
| cyanide ion* | 2.1 mg/dm3 |
| Na4[Fe(CN)6] ·3H2O | 6·103 mg/dm3 |
- total noncomplexed cyanide = [HCN] + [CN–]
The loss of dissolved oxygen is not a major problem for fish in the very small volume of the experiment, but it would probably be under natural conditions.
d) Are the experimental results and the toxicity data in agreement with the result of the experiment shown on the TV news show?
A little known fact about the pollution wave was that it also contained metals, primarily copper (which is hardly surprising for a gold mine). Copper is often present in our environment as copper(II), but it was present as copper(I) in the pollution wave because of the presence of cyanide ions.
e) Write the chemical equation for the reaction between copper(II) and cyanide ion.
An actual sample from the pollution wave had a pH of 7.5, its total cyanide content (including complexed, uncomplexed and protonated cyanide ions) was determined to be 26 ppm, its total copper content 21 ppm. The cyanide selective electrode gave a reading of 534.6 mV in this solution, and an electrochemical method showed that the concentration of free copper(I) is about 2·10–15 mol/dm3. Copper(I) forms complexes with cyanide ion in a stepwise manner up to a coordination number of 3. The formation constant of [CuCN] is negligible compared to that of the other two complex ions. Dissolved oxygen, the concentration of which was 8.00 mg/dm3, coexists with cyanocopper(I) complexes.
f) Is there any copper(I)-cyanide precipitate in the solution? (LCuCN = 3.5·10-19)
g) Determine the coordination number(s) of copper(I) complex(es) dominating in the sample studied. Estimate the stability constant(s) of the cyanocopper(I) complex(es).
The toxicity of copper(I) cyano complexes is very similar to that of NaCN; [Cu(CN)2]– has an LC50 value of 4.5 mg/dm3. To 100 cm3 of the sample from the pollution wave, 40.0 mg of solid FeSO4·7H2O was added. The cyanide selective electrode gave a reading of 592.3 mV in this solution.
h) Estimate the concentrations of various complexes in this sample. Is this solution expected to be toxic? Does this expectation agree with the experiment not shown on TV?
说明:官方答案步骤不完整且不易读懂,参考如下手写答案(credit goes to Bin)!
另外,前3小问比较有挑战性,电化学结合多重平衡(酸碱、络合和氧化还原等),需要用到两个物种的物料守恒!

IChO2008PP-Budapest-Q8L3
Numerous inorganic compounds undergo autodissociation in their liquid state. In liquid hydrogen fluoride (density, ρ = 1.002 g/cm3) the following equilibrium can describe the autoprotolysis:
3 HF = H2F+ + HF2–
The corresponding equilibrium constant is 8.0·10–12.
a) Calculate what fraction of the fluorine is present in the cationic species in liquid HF, supposing that only these three species are present in the system.
Various reactions can take place in liquid HF.
b) Write the equation of the reactions of liquid HF with the following substances: H2O, SiO2, acetone.
In water HF behaves as a medium-strength acid and dissociates only partially. The most important reactions determining the equilibrium properties of the solution are the following:
HF + H2O = H3O+ + F– (1)
HF + F– = HF2– (2)
The equilibrium constants of the two equilibria are
K1 = 1.1·10–3
K2 = 2.6·10–1
c) Calculate the analytical concentration of HF in a solution having a pH = 2.00.
In early studies of aqueous HF, equilibrium (2) was not considered. However, pH measurements, assuming only equilibrium (1) led to contradictions.
d) Show that, assuming only equilibrium (1), pH measurements can indeed lead to a concentration-dependent equilibrium constant for (1).
Two chemists wanted to determine the acidity constant of HF (K1) from one and the same solution with a known concentration. They measured the pH of the solution and then obtained a K1 value by calculation. However, the better chemist knew about equilibrium (2), while she knew that the other did not. So, she was surprised when they both obtained the same K1 value.
e) What was the concentration of the HF solution?
f) Calculate the equilibrium constant of the following equilibrium:
2 HF + H2O = H3O+ + HF2–
The dissociation equilibrium of a solute in a solvent can be significantly shifted by the addition of a suitable substance into the solution.
g) Propose three different inorganic compounds for increasing the dissociation of HF in water.
Suitable compounds can also shift the autodissociation equilibrium of HF in its liquid state by orders of magnitudes. A well-known such substance is SbF5.
h) Show how SbF5 shifts the autodissociation equilibrium of liquid HF.
The shift in the autodissociation also implies an important change in the Brønsted acidity of the solvent. In fact, the degree of solvation of the proton produced from the autodissociation essentially affects the Brønsted acidity of the solvent.
i) How is the Brønsted acidity of a given solvent determined by the extent of proton solvation?
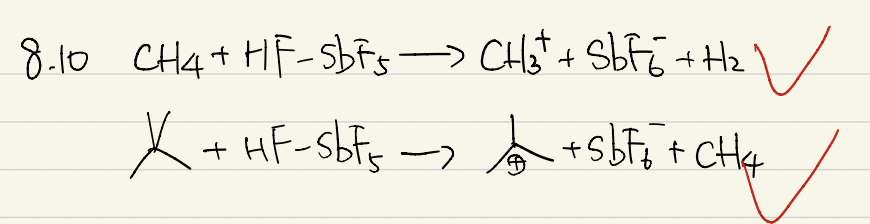
The mixture of HF-SbF5 belongs to the family of superacids, owing to their very high acidity. These acids are able to protonate very weak bases and thus have enabled the preparation of exotic protonated species. These, in turn, have opened new synthetic routes.
j) Formulate reaction equations for the reaction of methane and neopentane with the HF-SbF5 superacid. Note that in both cases there is a gaseous product.
说明:非水溶剂中的平衡综合题。
e)比较有趣且难度较大,充分利用物料守恒得到3个独立方程从而优雅的解出3个未知数。
原题可能需要稍微添加点解释文字以避免误判!

IChO2008PP-Budapest-Q9L3
Ammonium sulfide ((NH4)2S) is a widely used reagent in qualitative analytical chemistry. To prepare the reagent, hydrogen sulfide gas is bubbled through a 4‑5 mol/dm3 ammonia solution (excess) to produce ammonium sulfide, and then some water is added. The solution prepared in this way is almost never pure. It can contain either ammonia or ammonium hydrogen sulfide in excess when a lower or higher than stoichiometric amount of gas is absorbed.
- 10.00 cm3 of an ammonium sulfide reagent solution obtained above was diluted to 1.000 dm3. 10.00 cm3 of the resulting stock solution was transferred into a distillation flask and ~40 cm3 of water was added. Moreover, 20.00 cm3 of a 0.02498 mol/dm3 solution of sulfuric acid was added into the distillation flask. Then, 25.00 cm3 of 0.1 mol/dm3 cadmium nitrate solution was added into the collector flask (where the distilled components would condense).
- Approximately one half of the solution in the distillation flask was distilled into the collector flask [考虑提供温度?]. (In the collector flask, the formation of a yellow precipitate could be seen.)
- The content of the distillation flask was washed completely into a titration flask. After adding a few drops of methyl red indicator it was titrated with 0.05002 mol/dm3 NaOH solution. The volume of the titrant used to reach the equivalence point was 10.97 cm3.
- Bromine water was added to the solution in the collector flask (the precipitate dissolved), and the excess of bromine was removed by boiling the solution for 15 minutes. Bromine oxidizes all sulfur containing anions into sulfate ions. The hydrogen ions formed in the reactions in the collector flask were neutralized by 14.01 cm3 of 0.1012 mol/dm3 NaOH.
Calculate the exact composition of the reagent ammonium sulfide solution.
说明:这是一道挺难想明白整个过程的题,需要仔细分析每一步到底在做什么!
如果作为考题,需要适当增加说明文字或铺垫一些小问以引导思考,尤其是蒸馏出去的究竟是什么,如下所示:
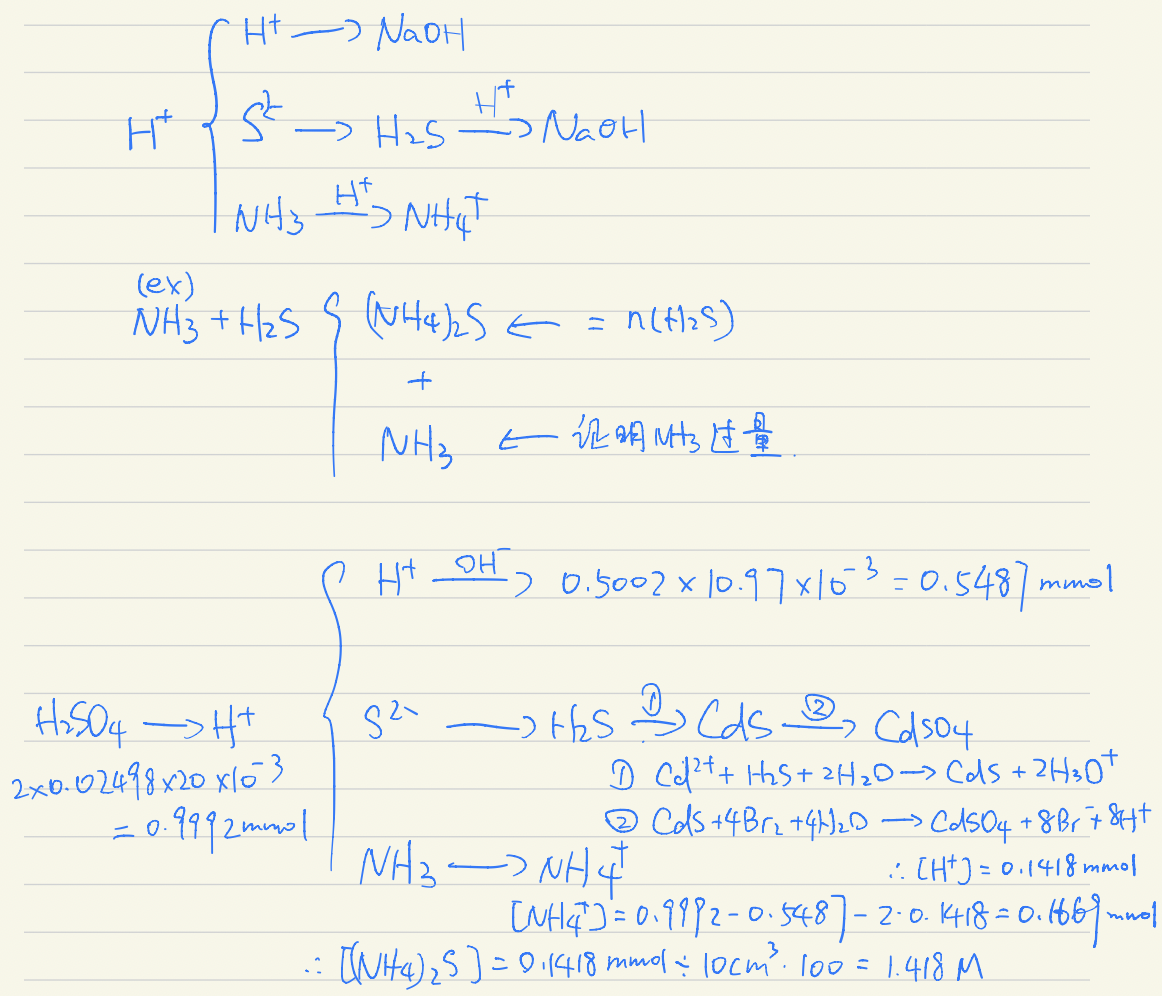
IChO2008PP-Budapest-Q12L2
The Fe3+/Fe2+ and the H3AsO4/H3AsO3 systems are important redox systems in analytical chemistry, because their electrochemical equilibrium can be shifted by complex formation or by varying the pH.
a) Calculate the standard redox potential, Eº3 of the reaction Fe3+ + e– → Fe2+.
The standard redox potential of the Fe3+/ Fe2+ system in 1 mol/dm3 HCl is 0.710 V.
b) Give an estimate for the stability constant of the complex [FeCl]2+.
Both Fe3+ and Fe2+ ions form a very stable complex with CN– ions.
c) Calculate the ratio of the cumulative stability constants for the formation of [Fe(CN)6]3– and [Fe(CN)6]4– ions.
d) H3AsO4 and K4Fe(CN)6 are dissolved in water in a stoichiometric ratio. What will the [H3AsO4]/[H3AsO3] ratio be at equilibrium if pH = 2.00 is maintained?
e) Are the following equilibrium concentrations possible in an aqueous solution? If yes, calculate the pH of the solution.
[H3AsO4] = [H3AsO3] = [I3–] = [I–] = 0.100 mol/dm3.
| Fe2+/Fe | Eº1 = –0.440 V |
|---|---|
| Fe3+/Fe | Eº2 = – 0.036 V |
| [Fe(CN)6]3–/[Fe(CN)6]4– | Eº4 = +0.356 V |
| H3AsO4 /H3AsO3 | Eº5 = +0.560 V |
| I2/2 I– | Eº6 = +0.540 V |
Errata:
The table contained standard redox potentials of the I2/2 I– system, while the solution used the value as if it belonged to the slightly different I3/3 I– system. We thank Szilárd Varga of Hungary for pointing this out.
IChO2008PP-Budapest-Q13L2
The solubility product of silver chloride is 2.10·10–11 at 9.7 °C and 1.56·10–10 at room temperature (25 °C).
a) Estimate the solubility product and the solubility (in mg/dm3) of AgCl at 50 °C.
Although AgCl is practically insoluble in water, it dissolves in solutions containing complexing agents. For example, in the presence of a high excess of Cl– ions, a part of the AgCl precipitate dissolves forming [AgCl2]– ions.
The equilibrium constant of the reaction Ag+(aq) + 2 Cl–(aq) AgCl2–(aq) is β = 2.50·105 at 25 °C.
b) Calculate the concentration of a KCl solution (at room temperature), in which the solubility of AgCl is equal to its solubility in water at 50 °C.
If a substance is present in a solution in various oxidation states, it cannot be determined directly by a redox titration. In this case, the sample has to be first reduced. For this purpose, so-called reductors are used. A reductor is a column, containing a strong reducing agent in the solid phase. An acidified sample is passed through the reductor, collected, and titrated with a strong oxidizing titrant of known concentration (for example KMnO4). The most common version is the so-called Jones-reductor that contains amalgamated zinc granules.
c) What reaction would take place if the zinc was not amalgamated?
d) Give the reactions that take place when the following solutions are passed through a Jones-reductor:
0.01 mol/dm3 CuCl2
0.01 mol/dm3 CrCl3
0.01 mol/dm3 NH4VO3 (pH =1)
e) Estimate the equilibrium constants of these reactions using the redox potentials in the table.
When a milder reducing agent is required, sometimes the Ag/HCl-reductor (containing porous silver granules and aqueous HCl) is used. This might seem surprising, since Ag metal is not a good reducing agent. Considering only the standard potentials, the reduction of Fe3+ to Fe2+ by Ag is not a spontaneous reaction.
f) Consider a silver rod that is immersed in a 0.05 mol/dm3 Fe(NO3)3 solution. Calculate the equilibrium concentration of the various metal ions. What percentage of Fe3+ ions has been reduced?
Now let us suppose that the reduction of Fe3+ with Ag is carried out in a solution that also contains 1.00 mol/dm3 HCl.
g) What reaction takes place in this case? Calculate the equilibrium constant of the reaction.
h) Calculate [Fe3+] at equilibrium if the initial concentration of Fe3+ was 0.05 mol/dm3.
i) Which of the following substances are reduced in an Ag/HCl reductor?
0.01 mol/dm3 CrCl3
0.01 mol/dm3 TiOSO4 (cHCl = 1 mol/dm3 )
| Eº / V | Eº / V | ||
|---|---|---|---|
| Cu2+/Cu | 0.34 | Cr3+/Cr | –0.74 |
| Cu2+/Cu+ | 0.16 | Cr2+/Cr | –0.90 |
| VO2+/VO2+ | 1.00 | Zn2+/Zn | –0.76 |
| VO2+/V3+ | 0.36 | TiO2+/Ti3+ | 0.10 |
| V3+/V2+ | –0.255 | Ag+/Ag | 0.80 |
| V2+/V | –1.13 | Fe3+/ Fe2+ | 0.77 |
IChO2011PP-Ankara-Q18L2
Drinking water may contain small amount of some contaminants that are harmful to living organisms. Iodine is used as a disinfectant for drinking water for the International Space Station Alpha. Aqueous I2 forms a number of inorganic derivatives, such as hypoiodous acid, HOI; iodate, IO3-; iodide, I- and triiodide, I3-. An equilibrium reaction takes place involving I2, I- and I3- in water according to the following equation;
I2(aq) + I-(aq) = I3-(aq)
When dichloromethane, CH2Cl2 is added to aqueous solution of iodine, I2 is distributed in water and CH2Cl2 phases according to the following equilibrium process. The equilibrium constant for the distribution is 150.
I2 (aq) = I2 (CH2Cl2)
a) For the homogenous equilibrium reaction which species acts as a Lewis acid?
b) One method for determining the concentration of I2 and I3- in a solution is the titration with a standard solution of S2O32-. An oxidation-reduction reaction takes place when I2 or I3- interacts with S2O32- yielding I- and S4O62-. Write the balanced equations for chemical reactions that take place during the titration of I2 and I3- with S2O32-. Indicate the oxidant and the reductant in each reaction? Give the oxidation state of S in Na2S2O3.
c) In order to determine the equilibrium constant of the reaction involving I2, I- and I3- in waterthe following experiments are performed at 298 K. When 50.0 mL of 0.010 M KI aqueous solution is added to 25.0 mL solution of I2 in CH2Cl2, two separate phases, aqueous and organic, are formed. Assume that there is no volume change upon mixing. In order to determine concentrations of I2 distributed in CH2Cl2 and aqueous phases, a 5.00 mL aliquot of the CH2Cl2 phase is diluted to 100.0 mL by addition of the solvent, CH2Cl2. The visible spectrum of I2 in the diluted solution, recorded in a 1.00 cm-cell, had a band with a maximum absorbance of 0.516 at 510.0 nm. The molar absorption coefficient, ε ofI2 in CH2Cl2 at 510 nm is 858 L·mol-1·cm-1. Calculate equilibrium concentrations of I2 in CH2Cl2 and aqueous phases.
d) In order to determine the equilibrium concentrations of I- and I3-, a25.0 mL aliquot is taken from the aqueous phase. To this solution, an excess amount of KI, namely 10.0 mL of 0.100 M KI solution, is added to avoid evaporation of I2. Then, the final solution is titrated with a 0.0100 Msolution of Na2S2O3. The end point is reached upon addition of 3.10 mL of Na2S2O3 solution. Calculate the equilibrium concentrations of I-, and I3-in the aqueous phase and the equilibrium constant at 298 K.
e) Calculate DfG°[I2(CH2Cl2)], if DfG°[I2(aq)] is 16.4 kJ·mol-1.
IChO2011PP-Ankara-Q17L2
Iodine is an essential trace element for life and is the heaviest element commonly needed by living organisms. At high temperatures an equilibrium between I2(g) and I(g) takes place.
The following table summarizes the initial pressure of I2(g) and the total pressure when the equilibrium is reached at the given temperatures.
| T (K) | 1073 | 1173 |
|---|---|---|
| P(I2) (atm) | 0.0631 | 0.0684 |
| Ptotal (atm) | 0.0750 | 0.0918 |
a) Calculate DH°, DG°and DS°at 1100 K. (Assume that DH° and DS° are independent of temperature in the temperature range given.)
b) Calculate the mole fraction of I(g) in the equilibrium mixture when the numerical value of Kp is the half of the total pressure.
c) Assuming ideal gas behavior for I2(g) and I(g), calculate the bond energy of I2 at 298 K.
d) Calculate the wavelength of radiation that must be used to dissociate I2(g) at 298 K.
e) In an experiment, when a sample of I2(g) is irradiated by a laser beam of λ =825.8 nm, at a rate of 20.0 J·s-1 for 10.0 s, 1.0×10-3 mol of I(g) is produced. Calculate the quantum yield for the dissociation process (i.e., the number of moles of I2 dissociated per mole of photons absorbed by the system).
IChO2011-Ankara-Q1L2
Nitrogen oxides, common pollutants in the ambient air, are primarily nitric oxide, NO, and nitrogen dioxide, NO2. Atmospheric nitric oxide is produced mainly during thunderstorms and in the internal combustion engines. At high temperatures NO reacts with H2 to produce nitrous oxide, N2O, a greenhouse gas.
2 NO(g) + H2(g)= N2O(g) + H2O(g)
To study the kinetics of this reaction at 820 °C, initial rates for the formation of N2O were measured using various initial partial pressures of NO and H2.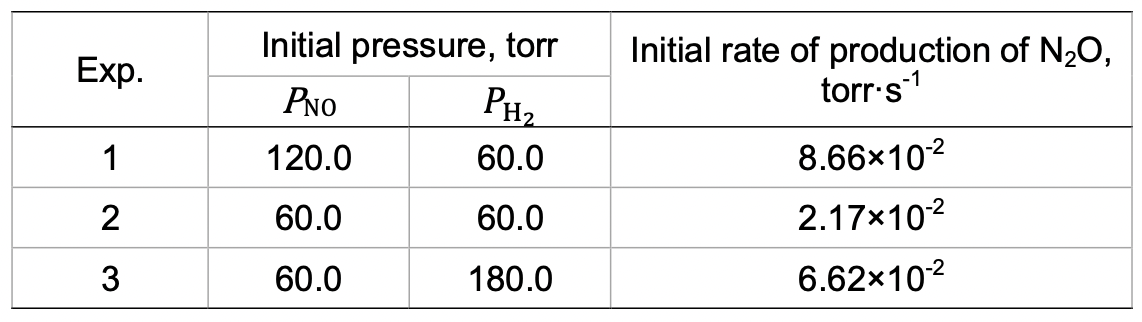
Throughout this problem do not use concentrations. Use units of pressure (torr) and time in seconds.
a) Determine the experimental rate law and calculate the rate constant.
b) Calculate the initial rate of disappearance of NO, if NO with a pressure of 2.00 ⋅ 102 torr and H2 with 1.00⋅102 torr are mixed at 820 °C.
c) Calculate the time elapsed to reduce the partial pressure of H2 to the half of its initial value, if NO with a pressure of 8.00⋅102 torr and H2 with 1.0 torr are mixed at 820 °C.
d) A proposed mechanism for the reaction between NO and H2 is given below: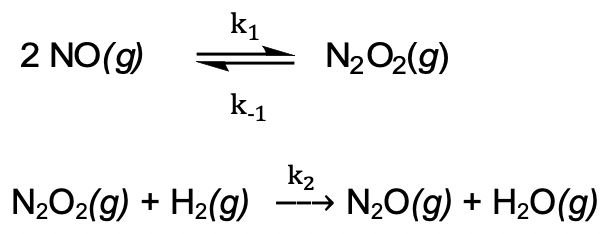
Derive the rate law for the formation of N2O from the proposed mechanism using the steady-state approximation for the intermediate.
e) Under what condition does this rate law reduce to the experimentally determined rate law found in Part a)? Tick the relevant answer.
A. If k–1 << k_2_p_H2 B. If _k–1 >> k_2_p_H2 C. If _k–1 > k_2 D. If _k_1 > _k–1
f) Express the experimentally determined rate constant k in terms of k_1, _k−1 and _k_2.
g) Select the schematic energy diagram that is consistent with the proposed reaction mechanism and experimental rate law.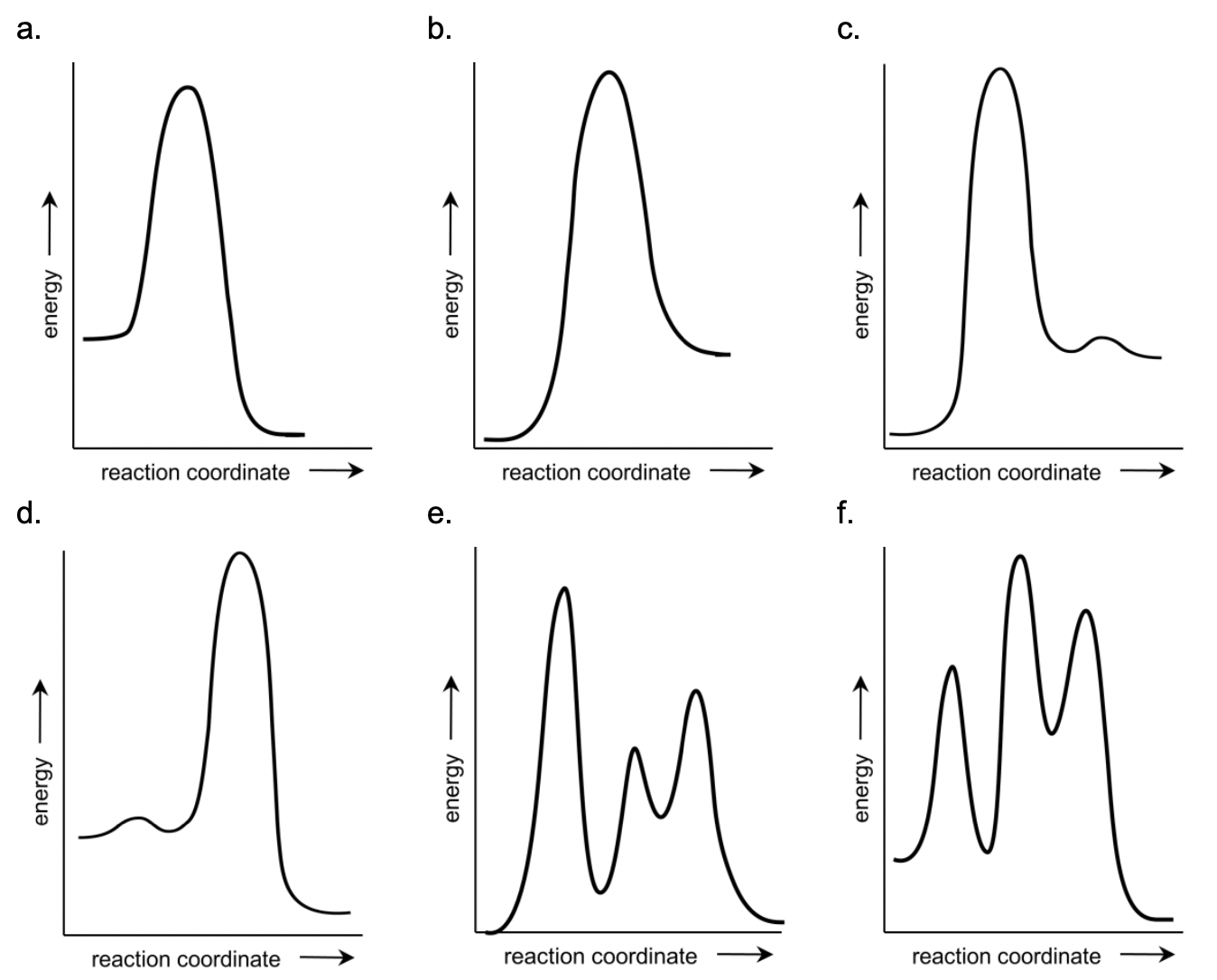
IChO2012PP-DC-Q11L2
Lead chromate has been widely used as a paint pigment, although this usage has been curtailed by environmental concerns in recent decades. Both components of this compound are hazardous to human health. Chromate is of particular concern because it is extremely mobile in groundwater. Therefore, humans can be exposed when they drink water from wells that are located at great distances from industrial sources of chromium.
a) Suppose that PbCrO4(s) in a landfill dissolves to equilibrium in a groundwater that has pH = 6.000. Using the following equilibrium constants, calculate the equilibrium concentrations of Pb2+, CrO42–, HCrO4– and Cr2O72–.
Quantities in parentheses () below are concentrations in mol·L–1. Assume that activity coefficients of all dissolved species equal 1.00 and therefore can be ignored. 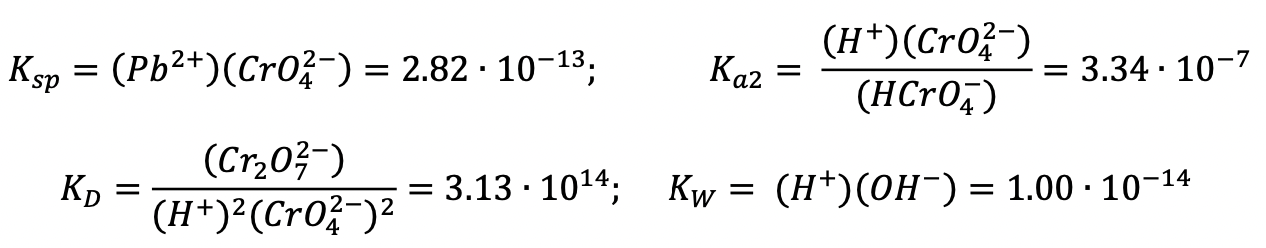
b) A toxicologist wishes to know at what total dissolved chromium concentration (CrT) the equilibrium concentration of HCrO4– equals that of Cr2O72– in the human stomach. Supposing that stomach fluid can be represented as a dilute solution with pH = 3.00, calculate CrT.
IChO2016-Georgia-Q2(part)L2
<br />Copper(I) oxide can be produced in a number of ways. Heating copper in air is a common method in the synthesis of semiconductor Cu2O. In a pure oxygen atmosphere, the three species containing copper (Cu(s), Cu2O(s) or CuO(s)) can potentially interconvert. <br />Suppose that the deltaf_H_o and _S_o data given for 105 Pa are independent of temperature: <br />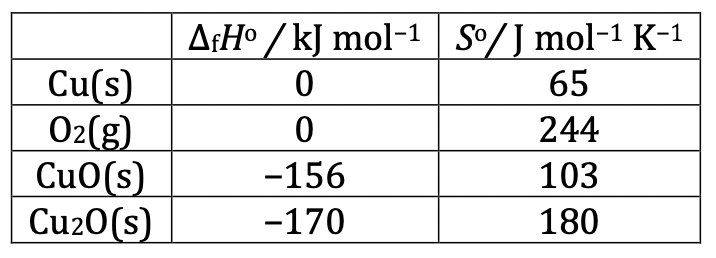 <br />**2.4. **Determine the temperature ranges, if any, of thermodynamic stability of copper and its oxides between 500 and 1500 K in a 105 Pa oxygen atmosphere. <br />Important data are given for 298 K. Use this temperature in the following calculations: <br /><br />One possibility for producing Cu2O is the anodic oxidation of copper. Electrolysis of an aqueous basic solution (e.g. NaOH) with a copper anode and platinum cathode can lead to formation of copper(I) oxide on the anode. <br />**2.5. **Write the half reaction equations for the electrode processes during the anodic production of Cu2O in NaOH solution with a platinum cathode and copper anode. <br />Electrolytic reduction of copper(II) ions in solution is another possibility. <br />**2.6.1. **Write the half reaction equation of the cathode process giving Cu2O in acidic medium. <br />Let us use 0.100 mol dm3 Cu2+ solution and carry out electrolysis with platinum electrodes. <br />**2.6.2. **What is the maximum pH at which the concentration of copper(II) can be maintained at 0.100 mol dm3? <br />If the pH is too low, reduction to metallic copper is preferred to the formation of copper(I) oxide. <br />**2.6.3. **What is the minimum pH at which the cathodic production of Cu2O in a 0.100 mol dm3 Cu2+ solution is still possible?
IChO2016-Georgia-Q3L3
Iodine deficiency is of special concern in Georgia because it occupies a region where iodine is scarce in soil and water. Iodine deficiency can be effectively and inexpensively prevented if salt for human consumption is fortified with small amounts of iodine. Methods for analyzing salt for iodine content are thus important. Current regulations in Georgia are that iodized salt must contain between 25-55 ppm iodine (1 ppm =
1 mg iodine/kg salt).
Most salt is iodized by fortification with potassium iodate (KIO3). Iodate content can be determined in salt samples using iodometric titration. In a typical procedure, 10.000 g of an iodized salt sample is dissolved in 100 cm3 of 1.0 mol/dm3 aqueous HCl to which
1.0 g KI has been added. The solution is then titrated with 0.00235 mol/dm3 aqueous sodium thiosulfate solution to a starch endpoint; this requires 7.50 cm3 of titrant.
3.1.1. Write a balanced net ionic equation for the reaction when iodate reacts with excess iodide in acidic solution.
3.1.2. Write a balanced net ionic equation for the reaction taking place during the titration with thiosulfate.
3.1.3. Calculate the iodization level, in ppm, of this salt sample.
A less common agent for iodizing salt is potassium iodide, which cannot be easily measured by iodometric titration.
One possible method for analyzing iodide in the presence of chloride is potentiometric titration. However, this method is not very precise in the presence of large amounts of chloride.
In this method, a silver wire is immersed in the solution (containing iodide and chloride) to be analyzed and silver ion is gradually added to the solution. The potential of the silver wire is measured relative to a reference electrode consisting of a silver wire in a 1.000 mol/dm3 solution of AgNO3. The measured potentials are negative and the absolute values of these potentials are reported. The solution to be analyzed has a volume of 1.000 dm3 (which you may assume does not change as silver ion is added), and T = _25 oC.
The results of this experiment are governed by three equilibria: the solubility of AgI(s) [_K_spI] and AgCl(s) [_K_spCl] and the formation of AgCl2(aq) [_K_f]. (Iodide also forms complex ions with silver but this may be neglected at the very low concentrations of iodide present in this experiment).
AgI(s) =Ag+(aq) + I(aq) _K_spI
AgCl(s) = Ag+(aq) + Cl(aq) _K_spCl
Ag+(aq) + 2 Cl(aq) = AgCl2(aq) _K_f
Below are shown the results of two experiments measuring the observed potential as a function of added number of moles of silver ion. Experiment A (solid circles) was carried out with 1.000 dm3 5 mol/dm3 iodide and no chloride ion. Experiment B (open circles) was done using 1.000 dm3 of solution containing 1 mol/dm3 chloride. 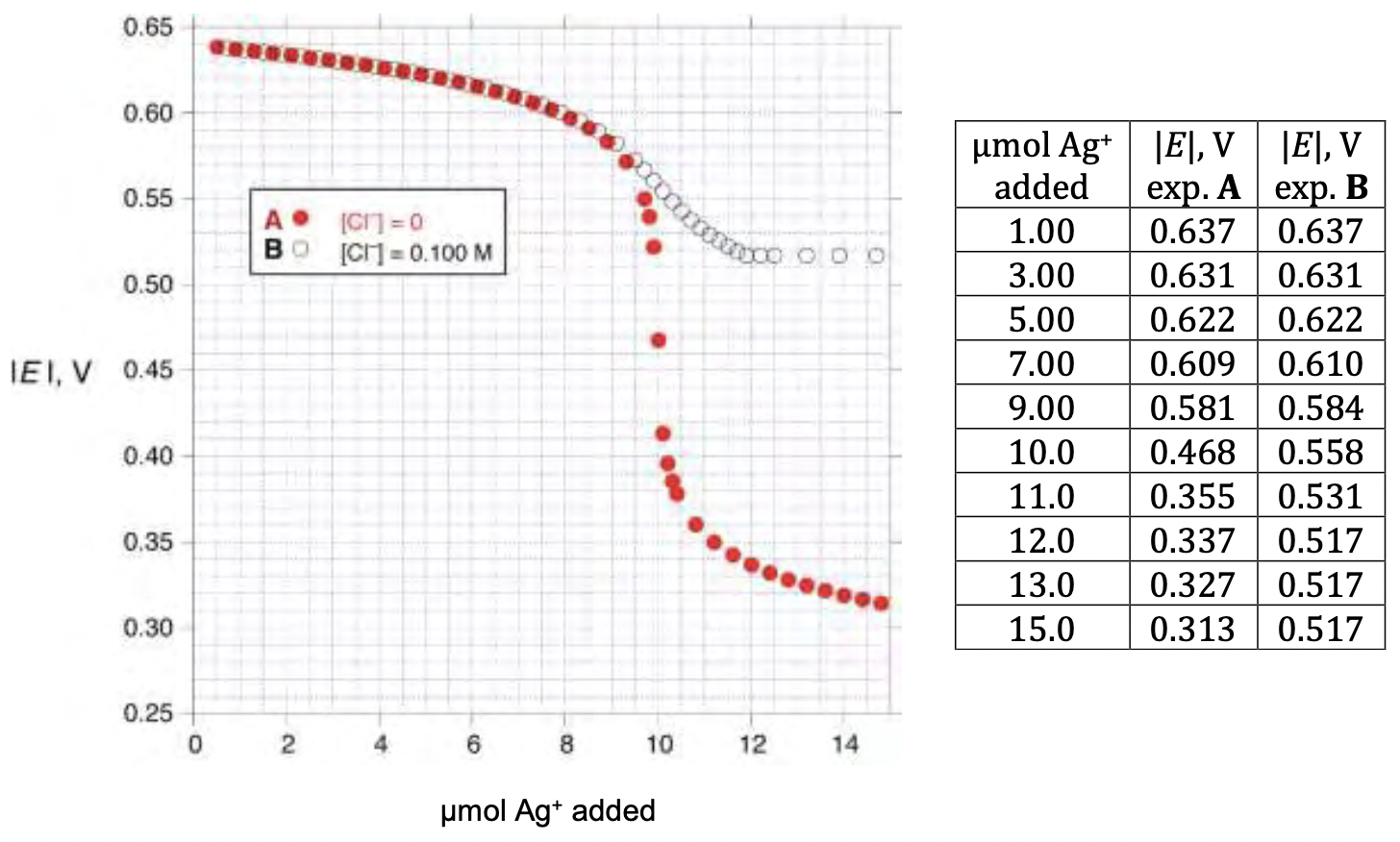
3.2.1. Select an appropriate data point from the experiments and use it to calculate the solubility product of AgI (_K_spI).
3.2.2. Select an appropriate data point from the experiments and use it to calculate the solubility product of AgCl (_K_spCl).
3.2.3. Select an appropriate data point from the experiments and use it to calculate _K_f. You may need to use values of _K_spI or _K_spCl to do this calculation. If you were unable to carry out the calculations in 3.2.1. or 3.2.2., you may use the arbitrary values of _K_spI = 15 and _K_spCl = 9 without penalty.
An analytical method that is more practical, because it is not sensitive to the presence of chloride, uses the Sandell-Kolthoff reaction. This is the reaction of H3AsO3 with Ce(IV) to give Ce(III) in acidic solution, which is strongly catalyzed by iodide ion.
3.3.1. Write balanced net ionic equations for the reaction of cerium(IV) with H3AsO3 in acidic solution, as well as reactions of cerium(IV) with a species containing the element iodine and H3AsO3 with a species containing the element iodine, that could reasonably account for the catalysis of the net reaction by iodide.
The reaction of Ce(IV) with H3AsO3 can be monitored by measuring the absorbance at 405 nm, as Ce(IV) is orange and absorbs significantly at 405 nm, while the other reactants and products are colorless and do not absorb appreciably. Three runs were carried out, all in 0.50 mol/dm3 H2SO4 using the following initial concentrations: 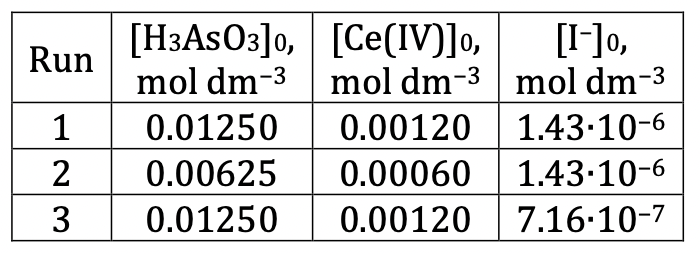
An analyst initiated the reactions by mixing the reagents in a cuvette. After a short variable delay absorbance measurements were started, with the first measurement recorded at _t=0 s. The data obtained are shown below: 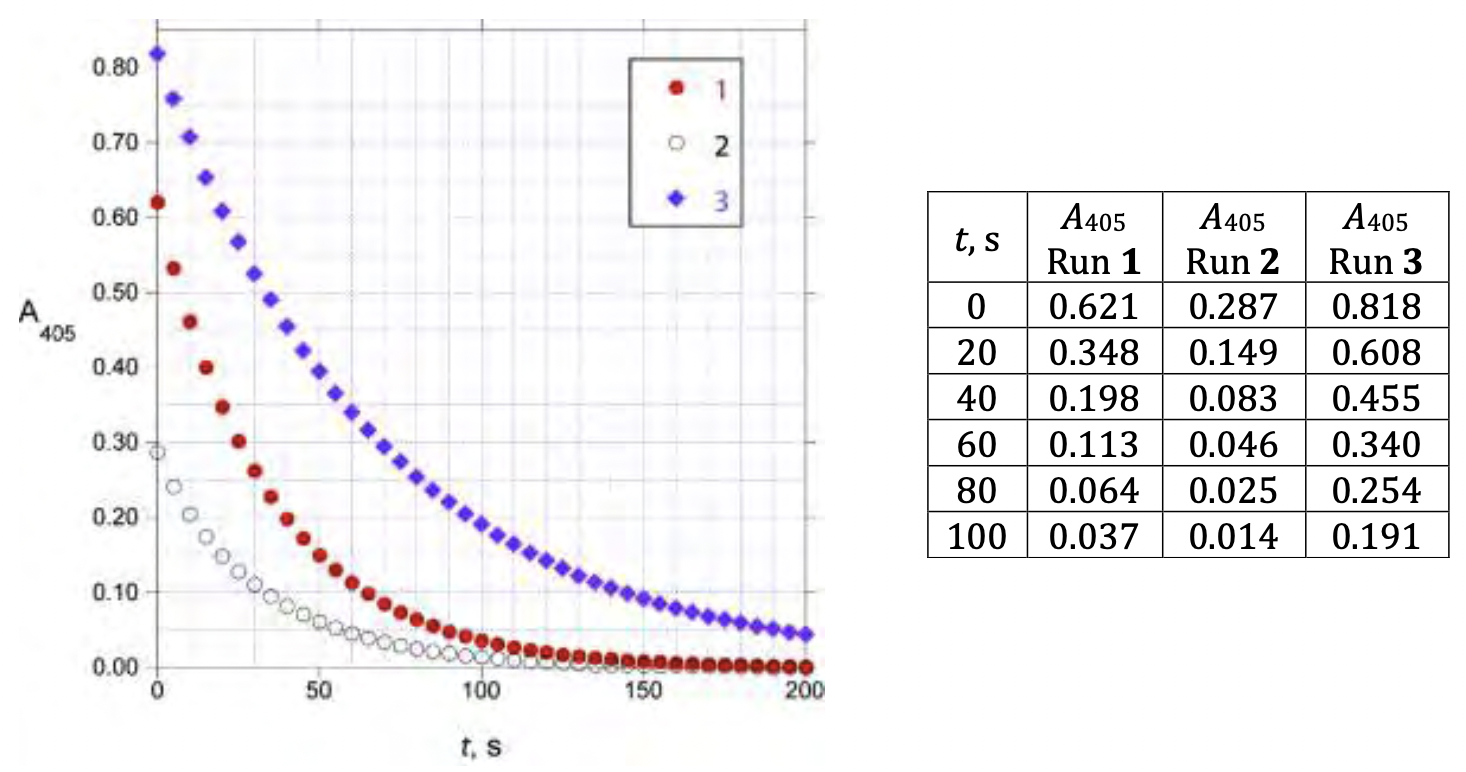 Under these conditions (0.5 mol/dm3 H2SO4, 25.0C), the rate law for the reaction can be written as
Under these conditions (0.5 mol/dm3 H2SO4, 25.0C), the rate law for the reaction can be written as
Rate = k[H3AsO3]m[Ce(IV)]n[I]p _where _m, n, and p _are integers.
3.3.2. Determine the values of _m, n, and p _and calculate the value of _k (be sure to specify its units).
A 1.000 g sample of iodized salt is dissolved in water to give 10.00 cm3 of solution. A 0.0500 cm3 aliquot of this solution is added to a mixture of 1.000 cm3 0.025 mol/dm3 H3AsO3 in 0.5 mol/dm3 H2SO4 and 0.800 cm3 0.5 mol/dm3 H2SO4. To this mixture is added 0.200 cm3 0.0120 mol/dm3 Ce(NH4)2(NO3)6 in 0.5 mol/dm3 H2SO4 and the absorbance at 405 nm is measured as a function of time at 25.0C:
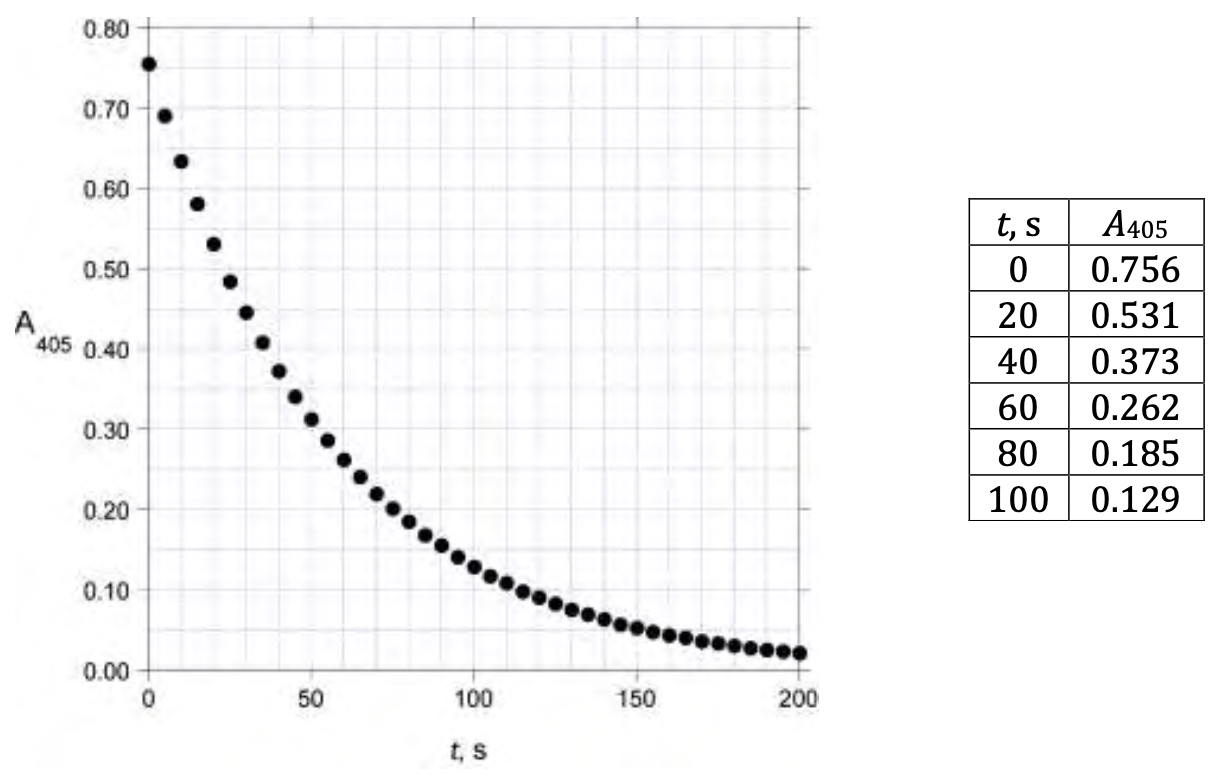
3.3.3. Calculate the iodization level, in ppm, of this salt sample.
<br /> <br />

