Outline
- Before you read
- Introduction to USNCO
- Catagory-based learning objectives
- Stoichiometry
- couting by weighing
- solution/gas stoichiometry
- emperical formula and mass percent
- hydrate/combustion analysis
- limiting reactants/yield
- mixture analysis (N2019P1-Q5)
- Colligative properties - VP/bp/mp/osmotic pressure
- electrolysis (N2017P1-Q3)
- Descriptive Chemistry/Laboratory
- color of hydrated ions
- acidity of salt solutions
- states of elements at room temperature
- dissolution heat of solids
- magnetism
- pressure measurement (N2017P1-Q11)
- seperation methods (filtration, distillation, extraction, chromatography, evaporation, recrystallization, etc.)
- solubility rules and color of precipitates
- typical experiments (melting point measurement, titrations, UV-vis spectrophotometry, etc.)
- indicators/error analysis of acid-base titrations
- volumetric glassware - precision
- aqueous reactions - neutralization, precipitation, complexation, redox, etc. (N2018P1-Q9)
- iodometry/KMnO4 (N2018P1-Q10)
- typical AP experiments - conductivity of H2SO4 + Ba(OH)2, Co2+ + Cl-, dilution of conc. H2SO4, fading of CV+
- Phases
- types and properties of solids
- misciblility of liquids
- IMFs and physical properties - bp/mp/VP/viscosity/surface tension/etc.
- Hydrogen bonds - intra/inter
- vapor pressure of liquids
- properties of gases and gas laws - Graham’s law
- phase diagrams - density of solids/liquids, sublimation, supercrital fluoid, critical point/triple point, etc.
- crystal structures - density, unit cell, formula
- Thermodynamics
- calorimetry (N2018P1-Q22)
- enthalpy change and Hess’s law - deltaHf, deltaHc, deltaHr
- first law of thermodynamics
- entropy
- Gibbs free energy and spontaniety
- K vs T - van’t Hoff equation (plot, N2018P1-Q23)
- VP vs T - Clayperon equation
- heat capacity - constant V and constant P (N2018P1-Q24)
- Kinetics
- relative rate
- rate constant and reaction order based on initial concentrations
- integrated rate law (plot) and half life (nuclear decay)
- energy profile of multistep reactions (concept of intermediate, catalyst, EA, etc.)
- Arrehnious law (k vs T)
- derivation of rate law by using SSA, RDS, PEA
- catalysis and enzyme kinetics
- Equilibrium
- LCP
- Kc vs Kp
- K-based calculations - RICE box
- pH calcuation
- buffer - double H equation
- acid-base titration curve - ep, sp, pH window, indicator and transition range
- precipitation equilibrium - Ksp, common ion effect
- multiple equilibrum - solubility vs pH, dissolution by complexation
- K vs T
- Redox/Electrochemistry
- oxidation states and balance of redox reactions
- Galvanic cell model (anode, cathode, salt bridge, etc.)
- standard reduction potential calculation (nFE)
- cell potential and cell notation
- Nernst equation - Ka/Ksp/Kf
- Faraday’s law
- deltaG vs K vs Ecell
- E-pH diagram
- Atomic Structures/Periodicity
- Hydrogen’s emission spectru and Bohr’s model
- quantum numbers
- peridicity - atomic/ionic radii, IE1, EA, EN
- orbitals and electron configurations - magnetism
- nuclear chemistry - alpha, beta, positrons, neutron capture/release
- Molecular Structures/Bonding
- polairy and dipole moment
- bond energy, bond length and bond order
- VSEPR and molecular geometry/bond angle
- molecular orbital theory
- acid strength
- Lewis structures/resonances/formal charges
- cis vs trans
- complex - geometry, isomerism, CFT, etc.
- new topics - bonds in SF4 (N2018P1-Q54)
- Organic Chemistry/Biochemistry
- functional groups and nomenclature
- acid-base properties of common functional groups
- substitutions vs eliminations
- isomerism
- chirality
- DBE
- free radical-based substitution - allylic and benzylic
- esterification and saponification
- aromaticity and AES
- chemistry of alkynes
- aldol and acetal formation
- pH and pI of amino acids/proteins
- biochemistry - cAMP, amino acids, proteins, DNA/RNA, enzymes
- biochemistry - saccharide, reducing sugar
- Stoichiometry
- Top questions in each catagory in USNCO 2015-19 (考虑去掉,毕竟很多题目会在前文中分析)
- Sample problems of USNCO 202x
-
Notes for writing
try to make each catogary independent as much as it can
Before you read
emphasize the book can be used for not only the USNCO, but also the other countries’ chemistry olympiad and even the IChO.
sample problems, anotated answers, frequently made mistakes, analysisIntroduction to USNCO
A timeline of the USNCO competition series (Local-National-Study Camp-IChO) including the style of each exam, the number of participants, and the difficulty level.
The criteria of the awarding methodCatogory-based Contents
Catogory 1 - Stoichiometry
[Pre-Reading]
(Zumdahls9e) Chp3 & 4.6, 4.11
(Atkins7e) Fund-L & Fund-M
<br />**[Learning Objectives]**<br />**1. Summary - Counting by Weighing**
[Conceptional Questions]
Recall the definition of relative atomic mass and moles, why the atomic mass has the same value as that of molar mass? If the Avagadro’s constant N = 3.01×10 mol, which is half of the actual value, which of the following parameter(s) will NOT change? Justify your answer. A. relative atomic mass B. molar mass C. molar volume of ideal gases D. molarity
[Extended Question]
How is the average relative atomic mass calculated? Taking chlorine for example, calculate the abundance of Cl and Cl considering the average relative atomic mass of chlorine is 35.45, respectively?
2. Percent Yield and Limiting Reactant
RICE box is a easy way to confirm the limiting reatant.
[Introductory Questions]
What does the coefficient in a balanced chemical equation mean, taking the ammonia synthesis below for example? For the ammonia synthesis reaction, what is limiting reatant if the initial masses of N and H are the same? What is the percent yield if 1/10 of the H is converted into ammonia (NH) when reaching equilibrium?
[Integrative Example]
A student reacted 1.73 g of salicylic acid (CHO) with 1.0 mL of acetic anhydride (CHO) to yield 1.50 g of acetylsalicylic acid (CHO) according to the reaction shown below.
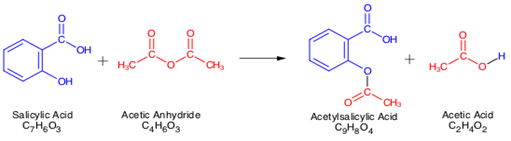
Given that the density of acetic anhydride is 1.08 g/mL, identify the a. limiting reagent; b. the theoretical yield (in grams) of acetylsalicylic acid, and [Answer = 1.9 g] c. the student’s percent yield in their experiment. [Answer = 79%]
3. Solution Stoichiometry and Volumetric Analysis
Molarity (c) is the conversion factor between the volume and moles of a substance in the solution.
For reaction:, where Z is the ratio of the coeffient of moles of B and that of A in the reaction.
Mass percetange (m%), molarity, molality (molal), mole fraction are differnt ways to express the concentration of solutions.
[Integrative Example – N2018-P1-Q12]
Which is the best way to prepare 500 mL of a 2.00 M solution of aqueous HSO from deionized water (M = 18.02, density = 1.00 g mL) and concentrated HSO (M = 98.08, density = 1.84 g mL)? (A) Weigh 98.1 g concentrated sulfuric acid into a 500-mL beaker, then slowly add deionized water to the beaker, with occasional swirling, until the liquid reaches the 500 mL mark. (B) Weigh 98.1 g concentrated sulfuric acid into a 500-mL volumetric flask, slowly add deionized water to the mark, and mix. (C) Weigh 98.1 g concentrated sulfuric acid into a 100-mL beaker, then slowly pour the HSO into a 500-mL beaker with about 250 mL deionized water in it. Pour this solution into a 500-mL volumetric flask and fill to the mark with deionized water and mix. (D) Weigh 446.6 g deionized water into a 500-mL volumetric flask, fill to the mark with concentrated sulfuric acid, and mix.
[Extended Question]
Taking concentrated HSO (98%) as an example, what parameter is needed to know in order to convert mass percentage into molarity (M)? How to convert molarity (M) into molality (molal)?
[Extended Question]
Sodium nitrite is used in the production of dyes for coloring fabrics, as a preservative in meat processing (to prevent botulism), as a bleach for fibers, and in photography. It can be prepared by passing nitrogen monoxide and oxygen gases into an aqueous solution of sodium carbonate. Carbon dioxide gas is another product of the reaction. In one experimental method, which gives a 95.0% yield, 225 mL of 1.50 M aqueous solution of sodium carbonate, 22.1 g of nitrogen monoxide, and a large excess of oxygen gas are allowed to react. What mass of sodium nitrite is obtained? [Answer = 44.3 g]
[Conceptional Question]
Recall the definition of molarity and molality, which one is temperature dependent? Why molality rather than molarity is used in colligative properties if solutions? Colligative properties of solutions are properties that depend upon the concentration of solute molecules or ions, but not upon the identity of the solute. Colligative properties include vapor pressure lowering, boiling point elevation, freezing point depression, and osmotic pressure.
Volumetric analysis is a widely-used quantitative analytical method. As the name implies, this method involves the measurement of volume of a solution of known concentration which is used to determine the concentration of the analyte.
[Integrative Example]
Sodium dithionite, NaSO, is an important reducing agent. One interesting use is the reduction of chromate ion to insoluble chromium(III) hydroxide by dithionite ion, SO, in basic solution. Sulfite ion is another product. The chromate ion may be presented in wastewater from a chromium-plating plant, for example. What mass of NaSO is consumed in a reaction with 100.0 L of wastewater having [CrO] = 0.0148 M. [Answer = 387 g]
5. Empirical Formula and Combustion Analysis
[Integrative Example]
If you become an organic chemist, you will almost certainly use combustion analysis at one stage of your work. Suppose you carry out a combustion analysis on 1.621 g of a newly synthesized compound, which is known to contain only C, H, and O. The masses of water and carbon dioxide produced are 1.902 g and 3.095 g, respectively. What is the empirical formula of the compound? [Answer = CHO] How to get the mass of oxygen in the compound?
[Extended Question]
In most of its ionic compounds, cobalt is either Co(II) or Co(III). One such compound, containing chloride ion and waters of hydration, was analyzed, and the following results were obtained. A 0.256-g sample of the compound was dissolved in water, and excess silver nitrate was added with all of the chloride ion precipitated. The silver chloride was filtered, dried, and weighed, and it had a mass of 0.308 g. A second sample of 0.416 g of the compound was dissolved in water, and an excess of sodium hydroxide was added. The hydroxide salt was filtered and heated in a flame, forming cobalt(III) oxide. The mass of cobalt(III) oxide formed was 0.145 g. a. What is the percent composition (each element), by mass, of the compound? b. Assuming the compound contains one cobalt ion per formula unit, what is the formula? [Answer = CoCl·6HO] c. Write balanced equations for the three reactions described.
6. Mixture Analysis
Settig up linear equations with two variables is the easiest way for mixture analysis. Usually, putting the moles of the substance as variables is practical in most cases.
[Integrative
Example] **
A 1.500-g sample of a mixture containing only CuO and CuO was treated with hydrogen to produce 1.252 g of pure cupper metal. Calculate the mass percent of CuO in the original mixture. [Answer = 40.% CuO]
[Extended Question – N2012-P1-Q11]
In an experiment to determine the empirical formula of magnesium oxide, a student weighs an empty crucible then adds a strip of magnesium metal and reweighs the crucible. The crucible and magnesium are heated with a burner flame, which ignites the magnesium and forms a gray-white solid. After cooling, the crucible and solid are reweighed and the data are analyzed to give an empirical formula of MgO. Which could account for the observed MgO result rather than the expected MgO? (A) Some of the magnesium reacts with atmospheric nitrogen to produce magnesium nitride. (B) A mixture of magnesium oxide and magnesium peroxide forms during combustion. (C) The piece of magnesium ribbon is shorter than recommended in the procedure. (D) The crucible and magnesium are heated longer than recommended in the procedure.
[Challenging Question – N2019-P1-Q4]
Magnesium metal burns in air to form a mixture of
magnesium oxide (MgO, M = 40.31) and magnesium
nitride (Mg3N2, M = 100.95). A 1.000 g sample of
magnesium ribbon is burned in air to give 1.584 g of the
oxide/nitride mixture. What percentage of the
magnesium is present in the form of the nitride?
(A) 9.00% (B) 11.0%
(C) 27.1% (D) 90.3%
<br />**[Practice
Problems]**
- The amount of arsenic, As, in a 7.25 g sample was determined by converting all the arsenic to arsenous acid (HAsO), and then titrating HAsO with 23.77 mL of 0.02144 M KMnO. What is the percentage by mass of As in the sample? An unbalanced expression for the titration reaction is HAsO(aq) + MnO(aq) = HAsO(aq) + Mn(aq) [Answer = 1.32%]
- An industrial by-product consists of C, H, O, and Cl. When 0.100 g of the compound was analyzed by combustion analysis, 0.0682 g of CO and 0.0140 g of HO were produced. The mass percentage of Cl in the compound was found to be 55.0%. What are the empirical and molecular formulas of the compound? [Answer = CHOCl]
- A 2.077-g sample of an element, which has an atomic mass between 40 and 55, reacts with oxygen to form 3.708 g of an oxide. Determine the formula of the oxide (and identify the element). [Answer = VO]
- You have two 500.0-mL aqueous solutions. Solution A is a solution of a metal nitrate that is 8.246% nitrogen by mass. The ionic compound in solution B consists of potassium, chromium, and oxygen; chromium has an oxidation state of 16 and there are 2 potassiums and 1 chromium in the formula. The masses of the solutes in each of the solutions are the same. When the solutions are added together, a blood-red precipitate forms. After the reaction has gone to completion, you dry the solid and find that it has a mass of 331.8 g.
a. Identify the ionic compounds
in solution A and solution B.
b. Identify the blood-red
precipitate.
c. Calculate the concentration
(molarity) of all ions in the original solutions. [Answer = 3.500 M CrO]
d. Calculate the concentration
(molarity) of all ions in the final solution. [Answer = 0.750 M CrO]
- Manganese is derived from pyrolusite ore, an impure manganese dioxide. In the procedure used to analyze a pyrolusite ore for its MnO content, a 0.533 g sample is treated with 1.651 g oxalic acid (HCO·2HO) in an acidic medium. Following this reaction, the excess oxalic acid is titrated with 0.1000 M KMnO, 30.06 mL being required. What is the mass percent of MnO in the ore? [Answer = 91.0%]
HCO + MnO
- H = Mn + HO + CO (not balanced)
HCO + MnO - H = Mn + HO + CO (not balanced)
- Zinc and magnesium metal each react with hydrochloric acid according to the following equations:
Zn(s) + HCl(aq) = ZnCl(aq) + H(g)
Mg(s) + HCl(aq) = MgCl(aq) + H(g)
A 10.00-g mixture of zinc and magnesium
is reacted with the stoichiometric amount of hydrochloric acid. The reaction
mixture is then reacted with 156 mL of 3.00 M
silver nitrate to produce the maximum possible amount of silver chloride.
a. Determine the percent
magnesium by mass in the original mixture. [Answer = 31.3% Mg]
b. If 78.0 mL of HCl was added,
what was the concentration of the HCl? [6.00 M]
[USNCO
Examples – N2012-Part
II-Q1]
[11%] The concentration of ethanol (C2H5OH) in aqueous solutions can be determined by reacting it with potassium dichromate (K2Cr2O7) in acid to produce ethanoic acid (CH3COOH) and Cr3+ ions.
- Write a balanced equation for this reaction.
Describe the color change observed during this reaction.
A standard solution of K2Cr2O7 is prepared in the following manner:
125 mL of H2O is placed in a 250 mL volumetric flask
70 mL of concentrated H2SO4 is added while swirling and cooling under running water
0.750 g of dried K2Cr2O7 is added and the solution is diluted to the mark on the neck of the flask with H2O.1. Account briefly for the order in which the liquid solution components are mixed (H2O, H2SO4, H2O) and for the fact theinitial mixture is cooled.
1. Calculate the [Cr2O72–] in the 250 mL solution.
0.600 mL of a popular mouthwash is diluted to 100. mL with H2O. When a 10.0 mL aliquot of this solution is titrated with the solution of K2Cr2O7 prepared in c., 20.25 mL of K2Cr2O7 are required.
- Calculate the number of moles of ethanol in 0.600 mL of mouthwash.
1. Determine the mass percentage of ethanol in this mouthwash (Assume the density of mouthwash to be 0.966 g•cm–3).
**
**

