Feb 1, 2021 [Slovakia_N2020_A1-1 (Part)]
Ammonium perchlorate and hydrazine are among the most widely used components of rocket propellants. Ammonium perchlorate serves as an oxidant in solid fuels, while hydrazine serves as a fuel in liquid state. The disadvantage of both compounds is their toxicity and impact on the environment. Therefore, a more environmentally friendly solution is currently being sought. One of the promising so-called The “green” alternatives are compound A, which decomposes on heat to nitrogen, oxygen and water (Reaction 1).
A(s) → 2N2(g) + O2(g) + 2H2O(l) (1)
This substance was first prepared in 1971 in the former USSR and was used, for example, in TOPOL ballistic missiles, because it does not produce easily observable flue gases. It was not synthesized independently in the United States until 1988. Compound A is a white inorganic salt that melts at 93 °C. Its cation contains two elements, its anion, which also contains two elements (let’s denote them X and Y), is composed of seven atoms. In this anion, all atoms of element X are in oxidation state of II, one atom of element Y is in oxidation state of I, and all other atoms of element Y are in oxidation state of IV.
[2 pts] 1. Write the molecular formula of compound A.
[18 pts] 2. Identify the cation and anion of compound A and draw their lewis structures. Provide the logical arguments that you use to identify.3. Thermal decomposition of ammonium perchlorate produces, in addition to nitrogen, oxygen and water, a corrosive colorless gaseous substance B(g). Write the equation of this reaction with the state of each substance labeled (reaction 2).4. Using the standard formation enthalpies (Δ_H_f°) listed below, calculate the standard reaction enthalpy (Δ_H_r°) of reactions (1) and (2).5. Which compound, A or ammonium perchlorate, releases more heat during thermal decomposition of 1.0 kg? Base your solution on a calculation.
https://en.wikipedia.org/wiki/Ammonium_dinitramide
possible revision direction: provide the N% and getting the molar mass through the colligative properties?learn form wcc and other USNCO Q1 or mocks?
~~may indirectly provide the decomposition reaction through absorbing different ~~
Solution: https://www.iuventa.sk/files/documents/2_olympiady/cho/56.%20ro%C4%8Dn%C3%ADk%202019-2020/ck/a/ch56ckateri.pdf
Feb 3, 2021 [CCE_N2020T1_Q28 (Part)]
Sulfuric acid is an important industrial chemical product. The key process in the production of sulfuric acid by contact method is the catalytic oxidation of SO2:
Answer the following questions.
(1) The energy change of those vanadium catalyst involved reactions are shown in Figure (a) below. Calculate the enthalpy change of the following reaction (not balanced):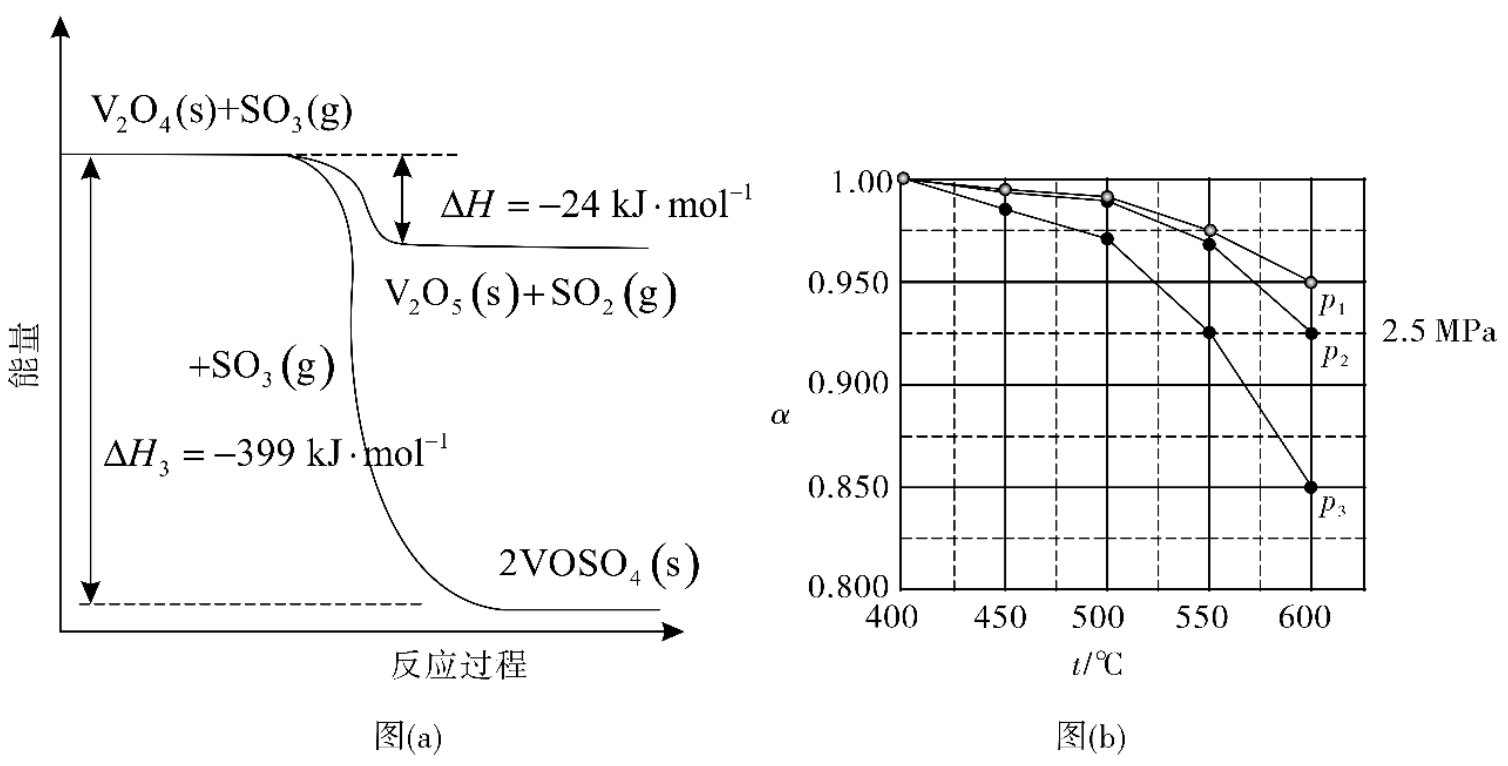
(2) If the initial mole fraction of SO2(g), O2(g), and N2(g) is 7.5%, 10.5%, and 82%, respectively, the percent yield of SO2 at equilibrium state α changing with temperature t/°C at different pressures (p_2 = 2.5 MPa, _p_1 and _p_3 = 0.5 MPa or 5.0 MPa) is shown in the Figure (b) above.
At _p = 5.0 MPa and 550 °C, α = __, justify your answer and list the factors to change alpha.
(3) A mixture with a composition of 2m% SO2(g), m% O2(g), and q% N2(g) [mole fraction] is added to the container, and reacts at temperature t and pressure p. If the percent yield of SO2 is α at equilibrium state, the partial pressure of SO2 = __, and Kp = _.
Feb 5, 2021 [Slovakia_N2020_A3-3 (Part)]
The structure of proteins has long been largely unknown to chemists, as is usually the case occurred in complex mixtures. Unless they were able to isolate into pure substances, theirs analysis was practically impossible because proteins consist of similar repeating units - amino acids. A major breakthrough was the discovery of reactions which could be used to label amino acids at the ends of a peptide chain. The labeling work and the gradual degradation of peptides from the end of the chain was discovered in 1955 by Frederick Sanger for the first time, which was used to solve the structure in a nature occurring protein - insulin. He was awarded the Nobel Prize for this discovery. Sanger’s labeling reagent (SR) can be synthesized from nitrobenzene in the following synthetic way: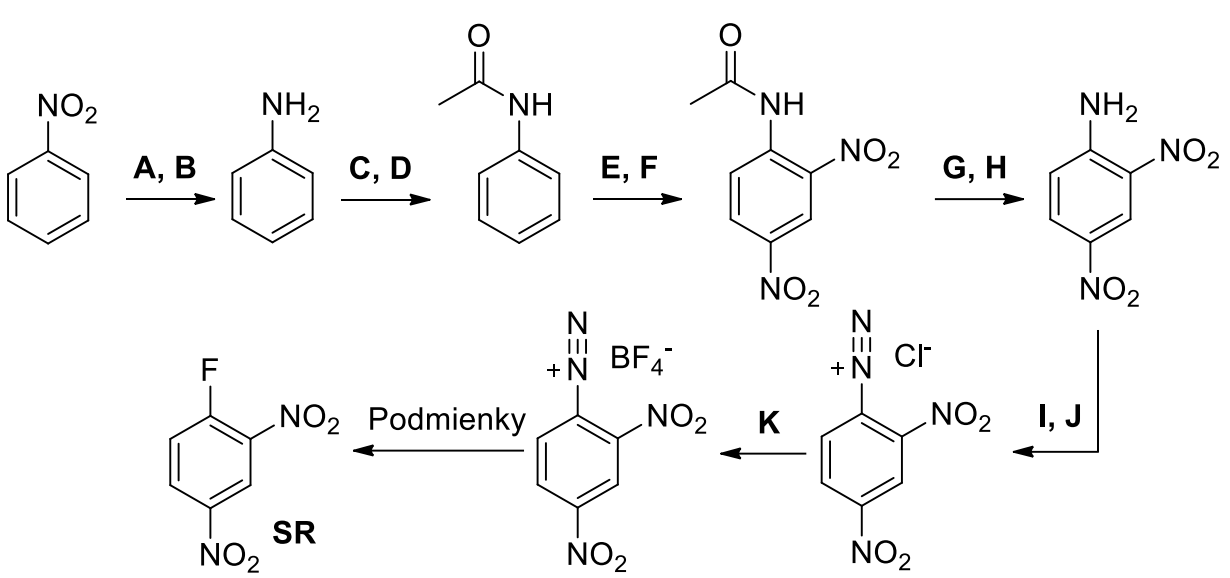 podemienky = condition
podemienky = condition
a) Add reagents A-K.
b) In the synthesis, two similar amines occur as intermediates - aniline and 2,4-dinitroaniline. However, the two substances differ significantly in their basicity. Indicate which of these substances is a stronger base. Justify your answer by using the resonant structure.
added: What is the purpose of step C, D?
c) Which condition is needed for the last reaction step (select ONE option)?
P1 - high pressure
P2 - high temperature
P3 - low temperature
P4 - presence of acid
d) Sanger’s reagent (SR) readily reacts with nucleophiles - for example methanethiol.
Write the mechanism of SR with methanethiol. What is the name of this mechanism?
Feb 7, 2021 [Keeler_Q20.5 (revised)]
The oxidation of methanoic acid by bromine
HCOOH + Br2 + 2H2O → CO2 + 2Br− + 2H3O+,
can be studied by setting up the reaction in an electrochemical cell. The potential (voltage) E generated by the cell is measured when the bromide ion in the reaction mixture is in much excess.
An experiment was designed to investigate the order with respect to Br2(a), methanoic acid (b) and acid (c).
rate = k[Br2]a[HCOOH]b[H3O+]c.
The reaction mixture is set up so that HCOOH and H3O+ are in much excess, making the rate law pseudo order.
The above expression for the rate law can be rearranged to
rate = k‘[Br2]a k‘ = k[HCOOH]b[H3O+]c.
The following data were obtained at 25 ◦C for a reaction mixture with the following initial concentrations: [Br2] = 3.0 × 10−3 mol dm−3, [HCOOH] = 0.10 mol dm−3, [H3O+] = 0.05 mol dm−3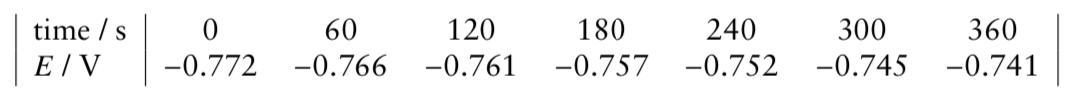
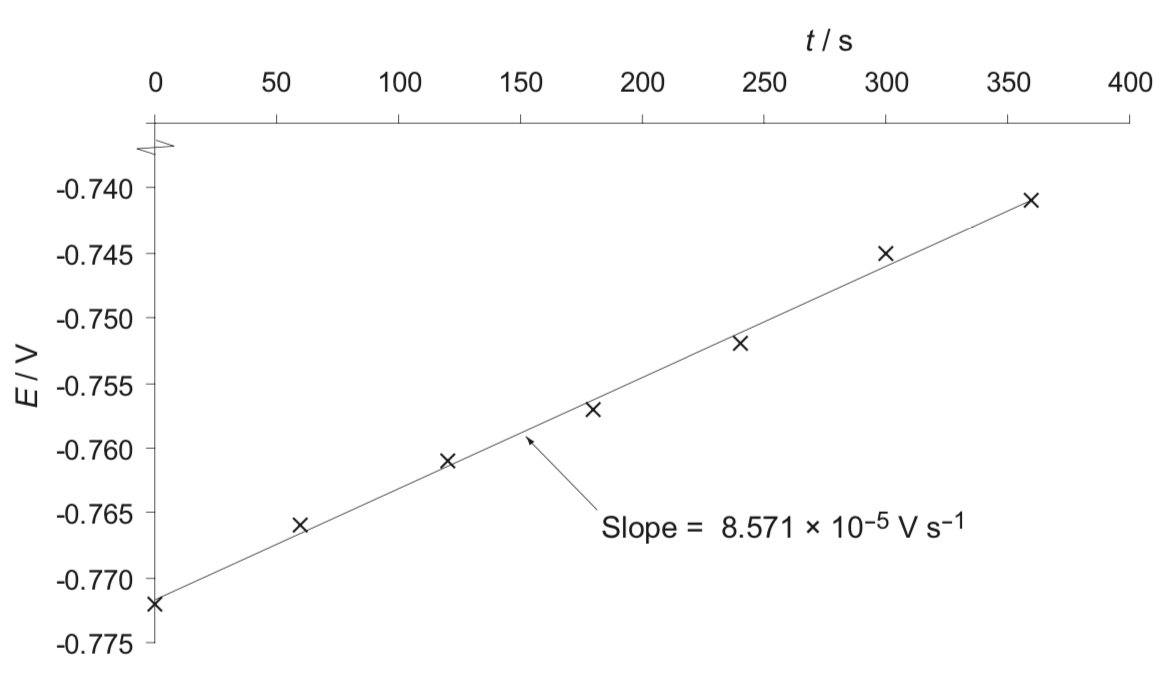
providing the figure is a better way, but why the slope is positive, [Br2] decreases with time, so does the E value?
may provide a new figure with the negative value?
(0) Derive the relationship between E and [Br2].
(i) Figure out the order with respect to Br2 (a) through calculation.
(ii) What is the observed rate constant k’?
Similar experiments, with different initial (excess) concentrations of methanoic acid and acid gave the following data (the value indicated by the * is obtained from the data table above)
(iii) Figure out what is the value of b and c, respectively?
(iv) Propose a reasonable mechanism for this reaction which is consistent with the rate law.
Feb 9, 2021 [Czech2019]
You have been called to search an abandoned building. In one of the rooms, you came across a box with six vials containing unlabelled solutions. You immediately marked the bottles with numbers 1-6 and wondered what they might contain. Ten pieces of chemical labels were also lying in the box. There must be labels of those six solutions among them! The following ten chemicals were marked on the papers:
NaOH, NaF, K2CO3, K2CrO4, AgNO3, MnSO4, FeCl3, KBr, HCl, ZnCl2.
Unfortunately, you didn’t have any other chemicals with you, just a few empty tubes. So you decided to identify solutes on the basis of interactions only.
57_cho_ae_dk_praxe_zadani_part.pdf

