Motivation
Most hardware involved in the IoT uses electrical energy to work.
The structure of an atom
All elements are made up of atoms.
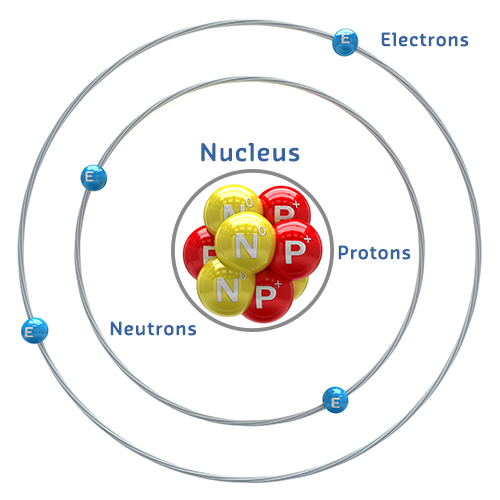
- Atoms have a nucleus which contains positively charged protons and neutrally charged neutrons, usually the same amount of each.
Orbiting around the nucleus are negatively charged electrons forming a cloud that occupies a volume 10000 times larger than the nucleus.
How the electricity occurs
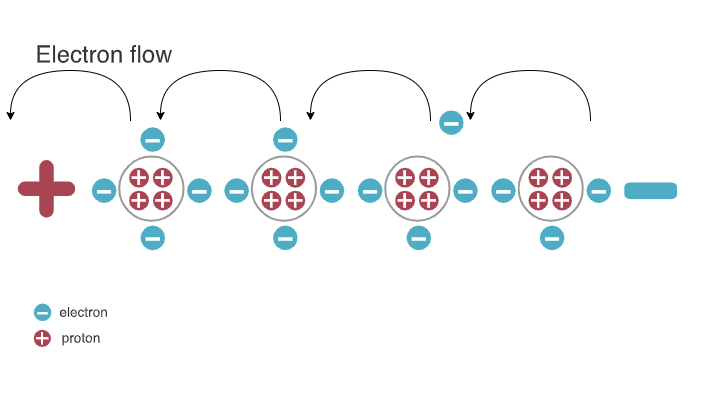
Materials can accumulate and lose electrons.
- A material that has accmulated an excess of electrons is described as negatively charged.
- On the other hand, when a matrial has a deficit of electrons, it is described as positively charged.
- Another word used to describle the state of charge is electrical potential.
- Things in nature tend to a state of equilibrium; hence electorns will flow wherever there is a difference in charge between two points.
- So electricity occurs when electrons in atoms jump from one atom to another
- The number of electrons flowing depends on the difference of charge between two points
- The unit for electric charge is the Coulomb.
- One Coulomb is equal to the electric charge of 6.24 X 1018, or 6.24 quintillion electrons
It is worth noting that although electrons physically move from a negatively charged point to a positively charged one, it is conventionally accepted that for the purpose of analysis and design, electric current flows from a positively charged to a negatively charged point.
Where does electricity come from?
The flow of electrons, can be produced in many ways.
However, since enery cannot be created or destroyed, only transformed, to produce electric enery weneed to first inject other forms of energy. Sometimes we burn fossil fuels and other non-renuewable resources to produce steam which, properly channelled, moves the rotating part of a generator. Sometimes we use solar and other renewable resources to produce electricity.
Current, voltage, resistance
Electric current
The flow of electrons jumpin between atoms from a negatively charged to a positively charged point is called electric current(I).
- Electrical current is measured in Amperes.
One Ampere represents the flow of one Coulomb of charge per second.
Vlotage
The difference in electric charge between two points is called voltage(V).
- The greater the charge difference(voltage) between two points, the higher the electrical current that will flow.
The unit for voltage is the Volt.
Resistance
Since all materials are made out of atoms, all of them have electrical properties.
- One important property is the ability called resistance(R) to conduct electricity.
- Good electrical conductors have low resistance, while materials that are not very good at conducting electric currents have high resistance.
The unit for resistance is the Ohm(Ω)
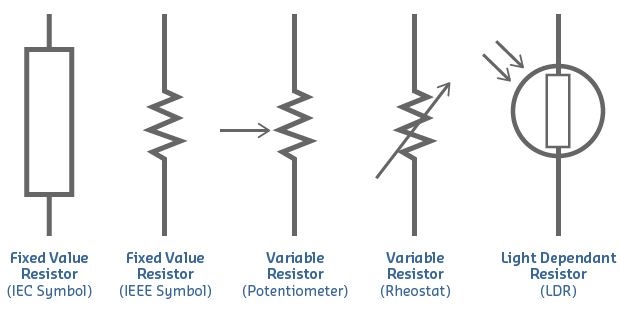
Resistors
In electrical circuits, resistance is often provided by resistors.
- Common symbols for resistors
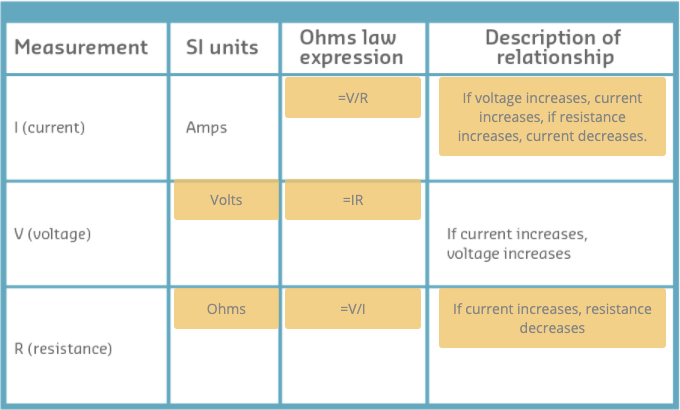
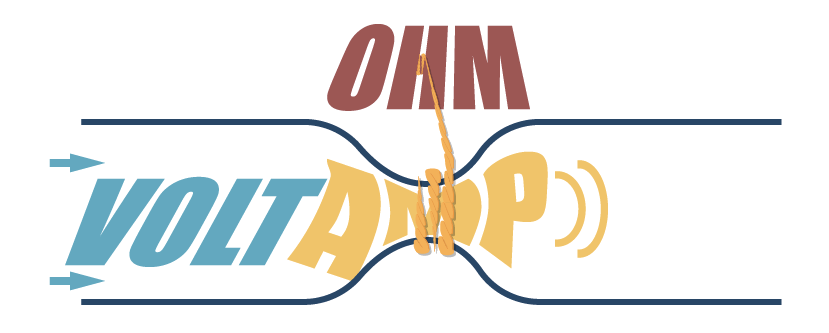
Ohm’s law
There is a very important relationship between voltage, current and resistance.
- One Volt can push a current of one Ampere through a resistance of one Ohm.
- That means the current flow through a conductor is directly proportional to the potiential difference(voltage) and inversely proportional to the resistance.
- _I = V/R, _Where electric current(I) eqals voltage(V) divided by resistance(R)
- The defferent expressions of Ohm’s law
Ohm’s wheel and electric power
- Power(P) is the rate at which energy is transferred or transformed over time;
- Power is measured in Joules per second or Watts.
Electric power is the product of voltage and current, or: P = V*I
Where power(P) equals voltage(V) multiplied by electrical current(I).
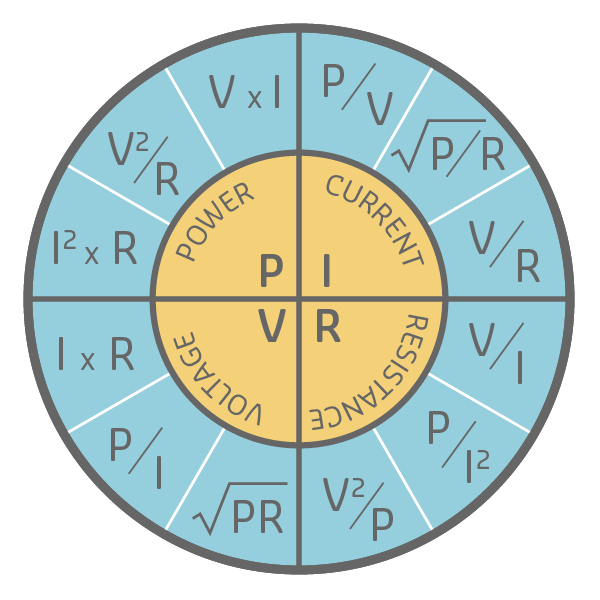
- The wheel shows 12 formulas three for each component - power, voltage, resistance and current

Capacitors and Inductors
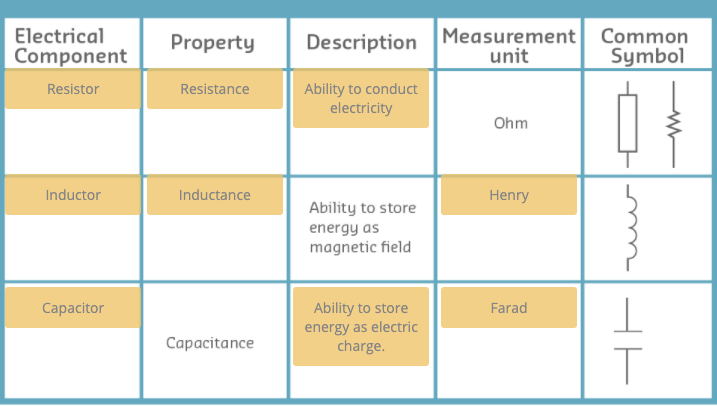 Capacitors
Capacitors
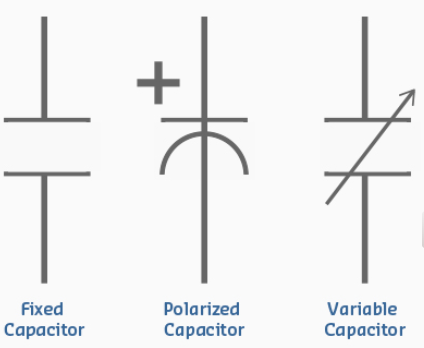
- These devices have the ability to store energy as electric charge.
- You may think of capacitors as tiny batteries than can be charged and discharged very quickly.
- Capacitors are useful to smoothen analogue signals and to decouple power hungry devices from the power supply.
- The ability to store charge is called capacitance.
- The unit of capacitance is the farad.
- however one farad is too big and commercial capacitors are measured in micro (10 ) or nano (10 ) farads.
Inductors

- These devices have the ability to store energy as magnetic fields when an electric current flows through them.
- Physically inductors are long wires wound into a coil around a core.
- The unit of inductance is the henry
But like capacitors one henry is too big and commercial inductors are rated in milli (10 ) or micro (10 ) henrys.
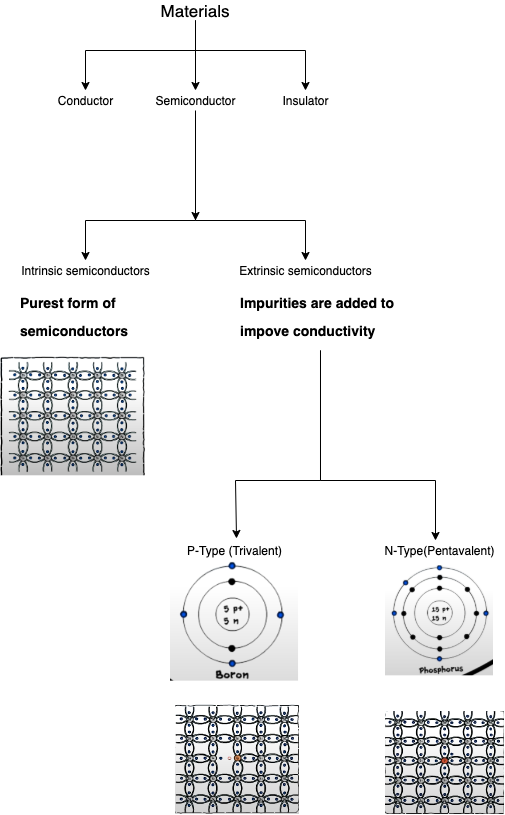
Semiconductors
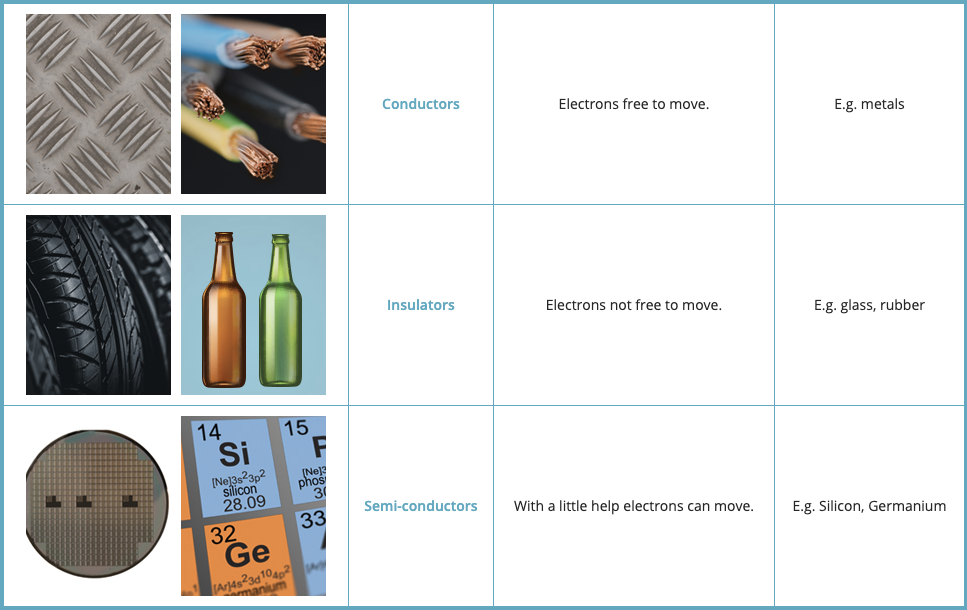
In some materials, electrons are free to move and they are called conductor. Most metals are very good conductors.
- In other materials, electorns are not free to move and they are called insulators. Rubber and glass are examples of good insulators.
- Other materials are neither good conductors nor insulators; they are called semiconductors. You may think of semiconductors as materials that need a little help to become good conductors.
- At an atomic level, insulators require enormous amounts of external energy for electrons to move long distances; while in conductors a difference in charge between two points is enough to get electrons moving.
- Under everyday conditions, conductors cannot turn into isulators, and vice-versa.
- With semiconductors, we need to apply a bit of external energy to get those electrons moving, turning the material into a conductor. But as soon as the external energy source is removed, the material goes back to its insulating state.
- The most common materials that present semiconductor properties are Silicon(Si) and Germanium(Ge).
- To make semiconductors useful - that is, to make it easy to control the amount of current flowing through them - they have to be ‘contaminated’ with other materials. These foreign ingredients are called dopants.
- Dopants are added to Silicon and Germanium to either increase or decrease the number of free electrons inside them.
- If dopants give the material an excess of electorns, then it is called negative(N-type) semiconductor.
- If dopants produce a deficit of electrons, then the material is called a positive(P-type) semiconductor.
- Examples of common dopants are Phosphorus for N-type, and Boron For P-type semiconductors.
Diodes
- A diode is a 2-terminal device that is very good at conducting electricity in one direction only.
- It is made with one part N-type and one part P-typ semiconductors.
- Its two terminals are called anode and cathode.
- Diodes become good conductors when the anode becomes more positive than the cathode by means of an external voltage supply.
- Structure and common symbols for diodes.

Diodes are commonly used as signal rectifiers or voltage references.
Transistors
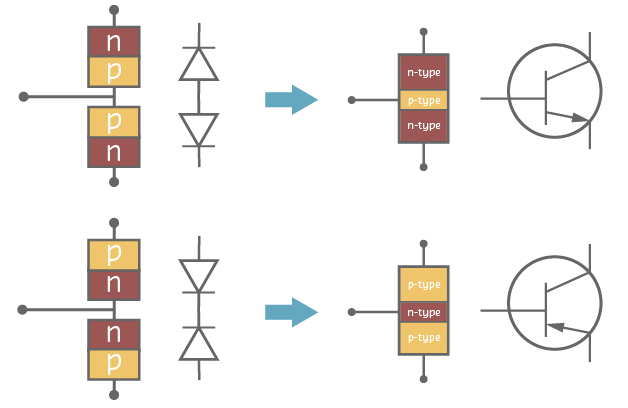
Bipolar Junction Transistor (BJT)
A Bipolar Junction Transistor is a 3-terminal device whose level of conductivity can be controlled with a small amount of current.
- It is made with three layers of semiconductor: N-P-N, or P-N-P. It’s terminals are called: Base, Collector and Emitter.

- Bipolar junction Transistors can be modelled with two diodes as shown in the following figures:
Field Effect Transistor(FET)
- Like BJTs, Field Effect Transistors are 3-terminal devices whose conductivity can be controlled with an external small voltage.
- The three terminals are called: Gate, Source and Drain.
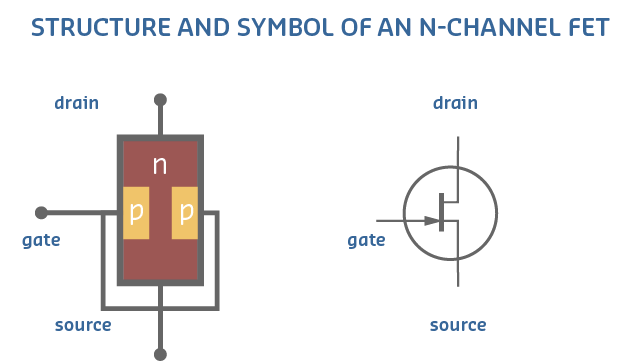
- Field Effect Transistors are very important because most of today’s electronic devices use them as their main building block.
- When FETs are in saturation or cut-off state, they consume practically no power because no current flows through them.
- Power consumption only occurs during transistions between the saturation and cut-off states.